What is Bakelite?
Bakelite is a polymer made up of the monomers phenol and formaldehyde. This phenol-formaldehyde resin is a thermosetting polymer.
We cannot deny the presence of polymers in our lives. We are surrounded by objects, most of which in some way or the other have a polymer associated with them. The ease of molding polymers into different shapes and their relatively low cost of production has been the main reason for their universal usage. As such, Bakelite is one of the commercially manufactured polymers that we witness in our daily lives.

Bakelite is the commercial name for the polymer obtained by the polymerization of phenol and formaldehyde. These are one of the oldest polymers that were synthesized by man. Phenol is made to react with formaldehyde. The condensation reaction of the two reactants in a controlled acidic or basic medium results in the formation of ortho and para hydroxymethyl phenols and their derivatives.
Preparation of Bakelite
When the phenol is taken in excess and the reaction medium is made acidic, the product of the condensation reaction obtained is acidic. Whereas, when the quantity of formaldehyde taken is more than that of phenol in the reacting mixture, and the reaction occurs in a basic medium, the condensation product is known as Resol.
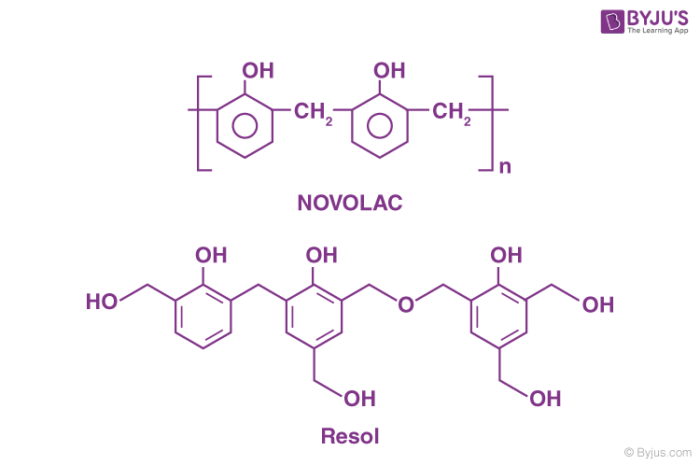
These intermediate condensation products are used as resins in different industries. Bakelite is obtained when Novolac is allowed to undergo cross-linking in the presence of a cross-linking agent. In general, phenol taken in excess acts as the cross-linking agent.
The preparation of bakelite involves several steps, as illustrated below.
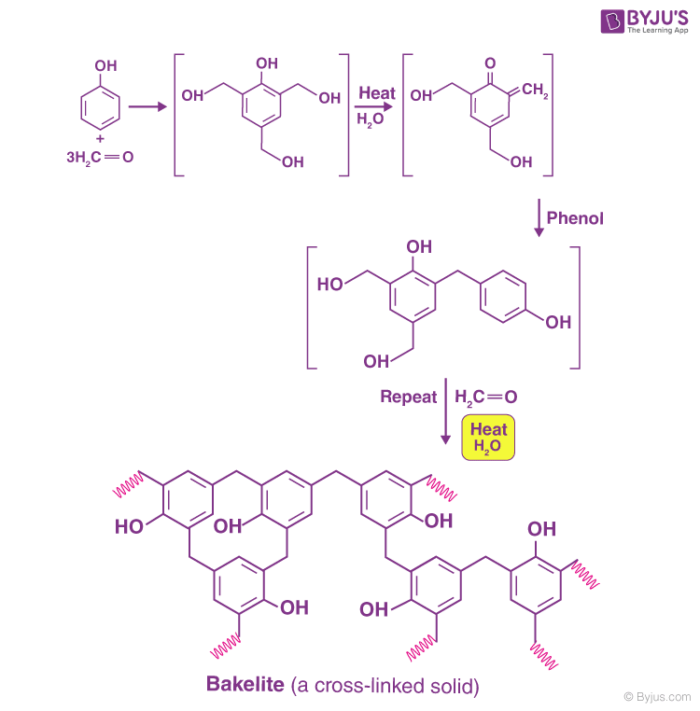
Initial methods of preparing bakelite involved the heating of formaldehyde and phenol in the presence of one of the following catalysts -zinc chloride (ZnCl2), hydrochloric acid (HCl), or ammonia (NH3).
Bakelite Structure
The cross-linked product of phenol and formaldehyde has the following structure.
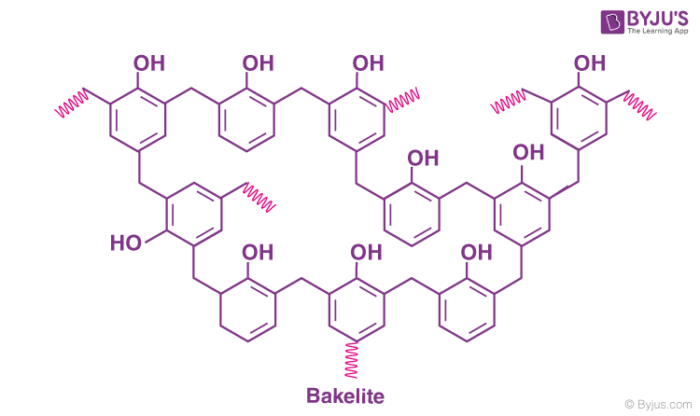
The chemical formula of bakelite can be written as (C6H6O-CH2OH)n.
What are the Desirable Properties of Bakelite?
Some important properties of bakelite are listed below.
- It can be quickly molded.
- Very smooth molding can be obtained from this polymer.
- Bakelite moldings are heat-resistant and scratch-resistant.
- They are also resistant to several destructive solvents.
- Owing to its low electrical conductivity, bakelite is resistant to electric current.
Uses of Bakelite
Now coming to the uses of Bakelite, since this element has a low electrical conductivity and high heat resistance it can be used in manufacturing electrical switches and machine parts of electrical systems. It is a thermosetting polymer and Bakelite has high strength meaning it basically retains its form even after extensive molding. Phenolic resins are also extensively used as adhesives and binding agents. They are further used for protective purposes as well as in the coating industry.
Further, Bakelite has been used for making the handles of a variety of utensils. It is one of the most common and important polymers that are used to make different parts of many objects.
Join BYJU’S or download our app to know more about polymers with the most simplified approach of learning.


Very useful app