Amides are a kind of functional group that cannot be skipped as the presence of a nitrogen atom in the compounds. Let us define amide along with its formation, types and structure.
Amide Functional Group
Functional groups provide the ability to identify and recognise a particular group of atoms which are part of a bigger compound. Functional groups range from alkanes to alcohols, which include amide. An amide is a functional group that consists of a carbonyl group and a nitrogen atom and can be derived from the various functional group known as carboxylic acid.
Types of Amides
When you visit an amide, you need to know a few points regarding nomenclature, whether it’s a name or structure. Amides are classified into three types based on their names: primary amine, secondary amine and tertiary amine. The differences are classified based on the position of the nitrogen atom linked to the carbon atom in a molecule chain. While naming a primary amide, you need to drop the end with ‘ic acid’ or ‘oic acid’ followed by an ‘amide’.
The secondary amide is named by integrating an N to show that nitrogen is linked to an alkyl group. An alkyl group is a kind of hydrocarbon chain that holds hydrogen and carbon atoms.
Amide Structure
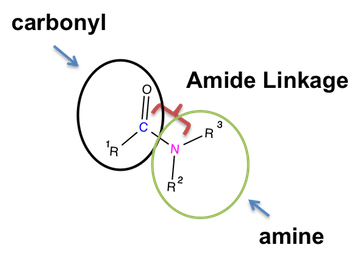
To remember an amide’s structure, all you need to know is a nitrogen atom should be present in an amide compound.
- First comes the carbonyl group, wherein a carbon atom is double-bonded to an oxygen atom.
- Second comes the amine group, wherein a nitrogen atom shares a single bond with R groups. You can consider R groups as substituents of other atoms or molecules to connect in a structure.
- Finally comes a single bond, which is also a characteristic functional group of an amide.
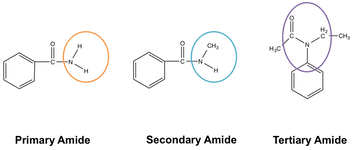
The above figure describes the structure of three kinds of amides. The positioning of the nitrogen atom is not common for three different amides. A nitrogen atom links to a single carbon atom in the case of a primary amide. The nitrogen atom links with two carbon atoms in the case of secondary amine. While in tertiary amide, the nitrogen links itself to three carbon atoms.
Applications and Occurrence
Amides are prevalent in technology as structural substances. An amide relation is developed easily, resists hydrolysis and grants structural rigidity. Polyamides and nylons are the most resilient materials. Various drugs are amides that include penicillin, LSD and paracetamol. Moreover, plant N-alkylamides consist of a broad range of biological functionalities.

Comments