Table of Contents
- What is a Displacement Reaction?
- Single Displacement Reaction
- Double Displacement Reaction
- Related Videos
- Frequently Asked Questions – FAQs
What is a Displacement Reaction?
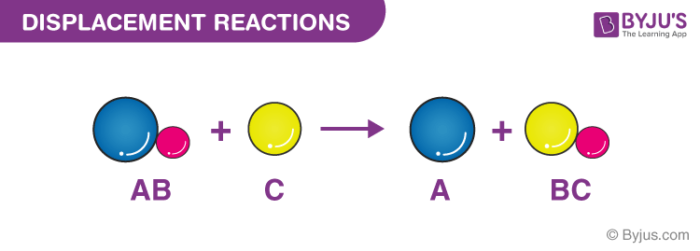
A displacement reaction is the one wherein the atom or a set of atoms is displaced by another atom in a molecule. For instance, when iron is added to a copper sulphate solution, it displaces the copper metal.
A + B-C → A-C + B
The above equation exists when A is more reactive than B.
A and B have to be either:
- Halogens where C indicates a cation.
- Different metals wherein C indicates an anion.
Recommended Video
Displacement Reactions

Single Displacement Reaction
A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound, i.e., one element is replaced by the other in a compound.
When chlorine is added in its gaseous form (or as a gas dissolved in water) to the solution of sodium bromide, the chlorine acquires the place of bromine. Since chlorine is more reactive than bromine, it displaces bromine from sodium bromide, and the solutions turn blue. The brown colour is the bromine that is displaced. If you notice the equation, you can see that the Cl and Br have swapped their original places.
chlorine + sodium bromide → sodium chloride + bromine
Cl2(aq) + 2NaBr(aq) → 2NaCl(aq) + Br2(aq)
Let’s analyse another chemical reaction.
Dissolve 0.5 gm of Silver nitrate in 10 ml of water in a test tube. A copper wire is then dipped in it and kept undisturbed for some time. The shining silver crystals are visible on the Copper wire. The solution becomes bluish as some amount of copper is developed. In the below reaction, the copper metal displaces silver from Silver Nitrate solution.
Cu(s) + 2AgNo3(aq) → 2Ag(s) + Cu(NO3)2(aq)
Double Displacement Reaction
Double displacement reactions occur when a part of two ionic compounds is exchanged and makes two new components. The pattern of a double displacement reaction is like this.
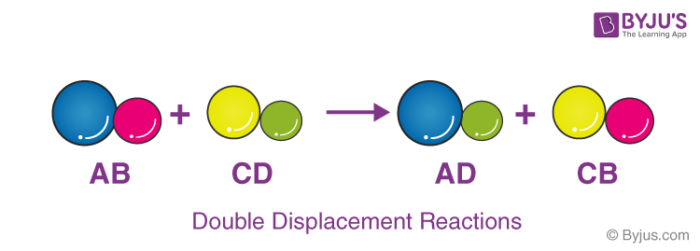
Double displacement reactions take place mostly in aqueous solutions wherein the ions precipitate and exchange of ions takes place.
For example, when a solution of barium chloride is mixed with sodium sulphate, a white precipitate of barium sulphate is formed rapidly. These reactions are ionic in nature. The reactants get transformed into ions when dissolved in water and an exchange of ions occurs in the solution and results in the formation of a product molecule.

Double displacement reaction between Barium Chloride and sodium sulphate
Recommended Video
Double Displacement Reactions with Examples

Frequently Asked Questions
What is called displacement reaction?
A displacement reaction is a type of reaction that replaces part of one reactor with another. Often known as a substitution reaction or metathesis reaction is a displacement reaction.
Why does a displacement reaction occur?
A single displacement reaction occurs when another element in a compound is replaced by an element. A metal only substitutes for a metal, and a nonmetal only substitutes for a nonmetal. Only a more reactive element in the compound with which it reacts can replace the other element.
What is double displacement give example?
Between two aqueous ionic compounds, there is a precipitation reaction to form a new insoluble ionic compound. Here is an example of a reaction to form (soluble) potassium nitrate and (insoluble) lead iodide between lead (II) nitrate and potassium iodide.
What is a Neutralisation reaction?
A neutralization reaction is a chemical reaction in which an acid and a base react to form a salt and water. The pH of the salt formed depends on the pH of the reacting acids and bases.
Are acid-base reactions double replacement?
There are two ionic compounds that exchange anions or cations in double substitution reactions. Reactions to neutralization occur when the reactants are acid and base, and reactions to neutralization are generally favourable as long as the reaction requires a solid acid and/or base.
To know more about different types of displacement reactions along with experiment procedure and results, you can visit us at BYJU’S.


What are the uses of displacement reactions??
Displacement reactions have applications in the production of steel, thermite welding, and in the extraction of some metals from ores.
What is displacement reaction
Displacement reactions involve a metal and a compound of a different metal. In a displacement reaction: a more reactive metal will displace a less reactive metal from its compounds. Click here learn more about Displacement reactions.
How does displacement reaction take place?
A displacement reaction, is a chemical reaction in which one element is replaced by another in a compound.