Ammonium Iodide is a chemical compound with a formula NH4I and can be manufactured in a lab by treating ammonium hydroxide with hydrogen iodide gas. It is easily soluble in water and ethanol. In moist air, it decomposes and turns yellow with the liberation of iodine. In this article, let us learn more about the ammonium iodide formula, its chemical structure along with its properties and uses.
Ammonium Iodide Properties
| Properties of Ammonium Iodide | |
| Name | Ammonium Iodide |
| Appearance | White crystalline powder |
| Chemical Formula | NH4I |
| Melting Point | 551 °C |
| Boiling Point | 235 °C |
| Molar Mass | 144.94 g/mol |
| Density | 2.51 g/cm³ |
| Solubility in Water | Soluble |
Ammonium Iodide Chemical Structure
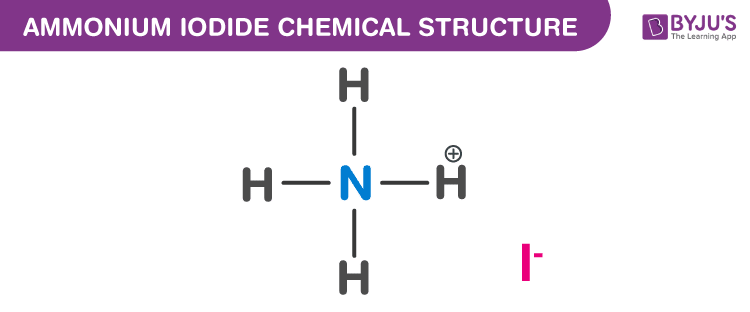
Ammonium Iodide Uses
- Used in photographic chemicals
- Used in medicines
Stay tuned to BYJU’s to learn more formulas of different chemical compounds.
Comments