Ammonium nitrite formula, also known as Ammoniumnitrit formula or Azanium nitrite formula is discussed in this article. It is highly unstable hence not used in its pure form. At room temperature, it decomposes to nitrogen and water. The molecular or chemical formula of Ammonium nitrite is NH4NO2. Ammonium nitrite is naturally formed in the air and it can be synthesized by the absorption of equal parts of nitric oxide and nitrogen dioxide in liquid ammonia. It can also be obtained by oxidation of ammonia with hydrogen peroxide or ozone. Also, it can be produced by precipitating lead nitrite or barium with ammonium sulfate, or potassium nitrite with ammonium perchlorate, or ammonium chloride with silver nitrite. The precipitate formed is filtered. The crystals obtained are colourless and dissolves in water.
Ammonium nitrite Formula Structure
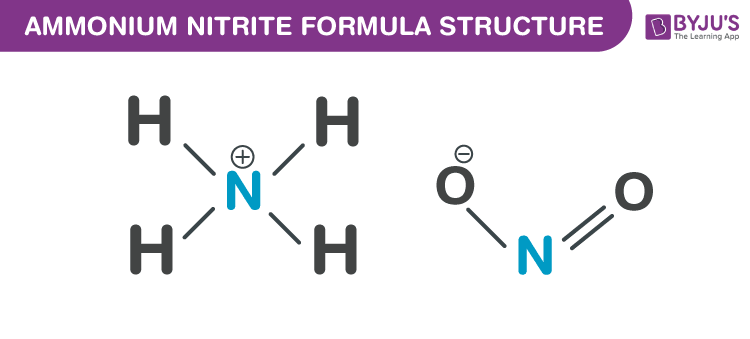
Properties Of Ammonium nitrite Formula
| Chemical formula | NH4NO2 |
| Molecular weight | 64.06 |
| Density | 1.69 g/cm3 |
| Appearance | Pale yellow |
| Melting point | Decomposes |
This inorganic chemical compound explodes if it is heated above 60 to 70 °C. The rate of decomposition is faster when dissolved in concentrated solution when compared to in dry crystal form.
To learn more about Ammonium nitrite formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Ammoniumnitrit for free.
Comments