The fizz in the club soda, the foaming effect in the washing soda are all due to carbonate ions. Carbonate is a salt of carbonic acid possessing the molecular formula CO32–. The main group of elements used as carbonates are alkali and alkaline earth metals. In their short piece of article, know more about the carbonate ion formula, its properties along with its chemical structure and uses.
Carbonate Properties
A few general properties of carbonate ion are listed below.
Physical State
- At room temperature, carbonates are solid
- Carbonates of group -1 and group-2 are colourless while carbonates of transition elements are coloured
- Group-2 carbonates are more covalent than group-1 carbonate
- The molar mass of the carbonate ion is 60.008 g·mol−1 and its conjugated base is bicarbonate.
Solubility
- Group-1 carbonates are soluble in water except for Li2CO3
- Group-2 carbonates are sparingly soluble in water
- Group-2 carbonates are fairly soluble in the solution of CO2
Thermal Stability
- Upon heating, carbonates decompose into oxide and carbon dioxide
- The thermal stability of group-1 and group-2 carbonates increase down the group.
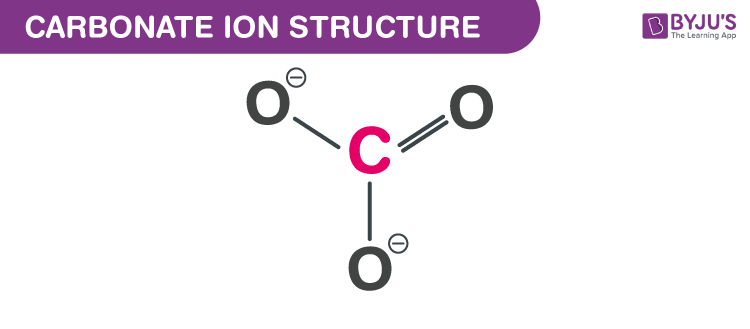
Carbonate Structure
It consists of a carbon atom surrounded by three oxygen atom in a trigonal planar arrangement. The Lewis structure of the carbonate ion has two single bonds to negative oxygen atom and double bonds to the neutral oxygen atom as shown below.
Carbonate Uses
- Carbonates such as potassium and sodium carbonates are used in washing detergents.
- Used to soften water
- Used as raw materials in the production of papers
- Used in glass manufacture
To learn more about such chemistry topics register to BYJU’S now!
Comments