Conformation is a property of atoms in which rotation of groups about a carbon-carbon single bond takes place. The rotation usually results in different spatial arrangements of the atoms. Likewise, conformational isomers are a type of stereoisomers where we can convert one isomer to another one, just by rotating it around a single bond. Let us further understand the concept with the help of an example:
Table of Contents
Conformers of Butane
We will take the example of conformers of butane. We will look at the structure of butane and draw its Newman projection. We will get the structure as given below:
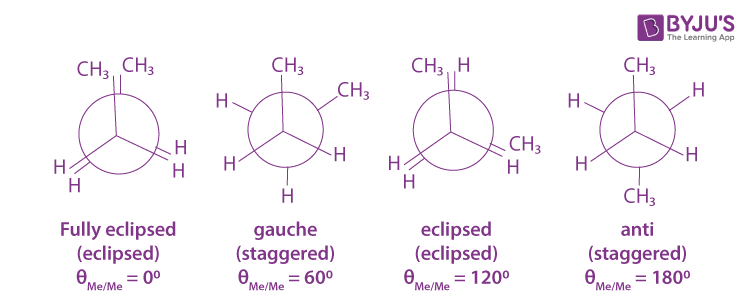
Butane is an alkane with the presence of C-C bonds. Normally, when we rotate the molecule of butane at the axis of the C-C bond, it shows different conformation isomerism. Generally, Butane has four conformation isomers which are fully eclipsed, gauche, eclipsed, and anti butane conformational isomers. Lets us discuss these isomers below.
-
-
-
- When we look at the chemical structure of butane, we can see that it has two substituents which are methyl groups attached to the two end carbon atoms. The methyl group is comparatively larger than hydrogen atoms. However, we can also see that everything on the front side is eclipsing with the structure in the backside. One methyl group is present in front of the other methyl group, and the other hydrogen atoms are kept at the back of other hydrogen atoms. In short, the structure is completely eclipsing or they are fully eclipsed.
- Now, if we rotate the front methyl group by 60° or when the dihedral angle is 60° then there is a formation of gauche or staggered conformation. It basically has the presence of identical groups at 60 degrees from one another. This type of conformation is more stable as there is a little steric hindrance between the same molecules.
- If we make the dihedral angle as 120° then this form of isomer is also known as eclipsed conformation. However, this eclipsed form is quite different from the previous fully eclipsed state. The basic difference is that in this the methyl group is eclipsing the hydrogen atom and not the methyl group.
- On rotating the isomer at an angle of 180° the two methyl groups are found to lie exactly opposite to each other. This conformation is known as staggered conformation or anti conformation.
-
-
Usually, each conformer is also interconverted to the same forms by rotating the isomer around the central carbon single bond. Eclipsed conformer can be converted back into gauche conformation by rotating it 60 degrees and when an eclipsed is rotated by 180 degrees it forms anti- conformer. Rotation of anti butane by 120 degrees again leads to the formation of the gauche conformed.
Ranking of Conformation Isomers
The ranking of the conformational isomers is given below taking into account the energy levels from lowest to highest. It is given as;
-
-
-
- anti
- gauche
- eclipsed
- fully eclipsed
-
-
This can also be visualized using an energy diagram.


Comments