Requirements
The materials that were used to test in this project included sand, water, salt, oil, soil and tape.
Method
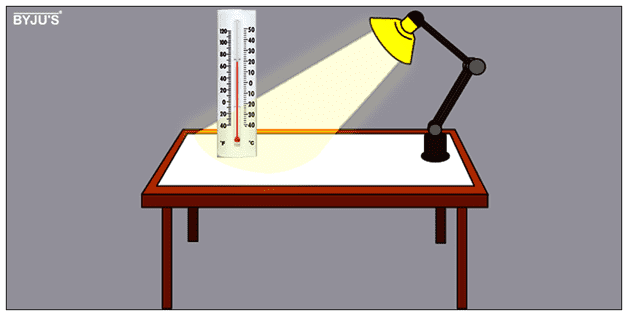
Take a container and place a material inside it along with two thermometers, one at a depth of two centimeters each and another on the surface. Now turn on the lamp for one hour. Place this lamp at a distance of a few centimeters over a material. Check the temperature every five minutes for each thermometer. After completion of one hour of time span, turn off the lamp. Again, record the temperature every five minutes. It was conducted in three intervals for each material.
Results
After analysing the test results, it was evident that the soil was loosened when it absorbed heat. Moreover, it did not absorb much heat compared to other materials used in this experiment. The water absorbed a small amount of it but it was not released; it kept in it. The oil absorbed a lot of heat compared to other materials, and it ended by losing lots of it but not at the rate of earth materials.
Conclusion
This working science model for class 10 illustrates different types of materials that absorb and releases heat. By this, one may try to make climate predictions. Suppose you are staying in a city beside the beach; it will definitely have a good weather as water keeps the heat. The weather over there is neither too hot nor too cold.
Comments