Table of Contents
Characteristics of MatterRecommended VideosDiffusionFactors Affecting DiffusionClassification of MatterBose Einstein Condensates
Our cosmos is made up of matter. The matter is stated as any substance that has mass, takes volume and may be comprehended by the senses. Exceptions like heat, electrical energy, light energy, sound energy, magnetism, vacuum, a shadow. These are not matter because these have no mass and do not take up space.
The matter is composed of small constituent parts. The units of matter are very minute. We cannot see them even with a high-power microscope.
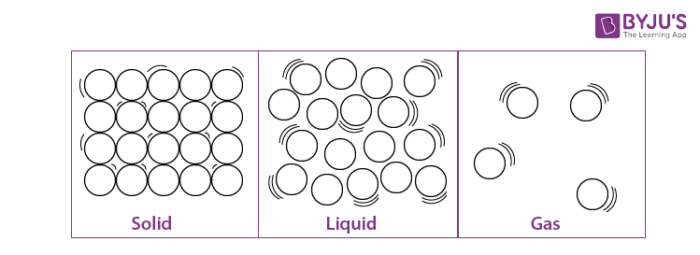
Characteristics of Matter
The Characteristics of Matter are listed below.
- The matter is created out of small particles.
- The particles have intermolecular spaces amidst them.
- The particles of matter are locomotive in nature because they have kinetic energy. The motion of the constituent particles intensifies with a surge in temperature.
- The bits in matter draw each other, but this reciprocal force of pull is operational only when the particles are very close by to each other. In solids, these units are narrowly crammed, and hence they have superior intermolecular force attraction although, in gases, the groups are lightly held. Therefore, they have feeble forces of attraction.
Recommended Videos
Matter in Our Surroundings – States of Matter Quiz

Matter Around us

Diffusion
The intermingling of the particles of two or more materials on their own is called diffusion.
Diffusion is quicker in gases. The speed of diffusion is different in different gases. Lighter gas intermingles at a faster speed than denser gases. Liquids, as well as solids, undergo dissemination. However, the speed of diffusion in solids is pretty low. As particles of liquids move slowly, diffusion in liquids is slower than in gases.
Factors Affecting Diffusion
1. Density
The rate of diffusion is inversely relative to the mass of a liquid or a gas. Greater the density, the smaller is the rate of diffusion.
2. Temperature
The speed of diffusion is directly proportionate to the temperature. As the temperature increases, the kinetic energy of the constituent units upsurges, and they travel with greater speed resulting in an amplified rate of diffusion.
Classification of Matter
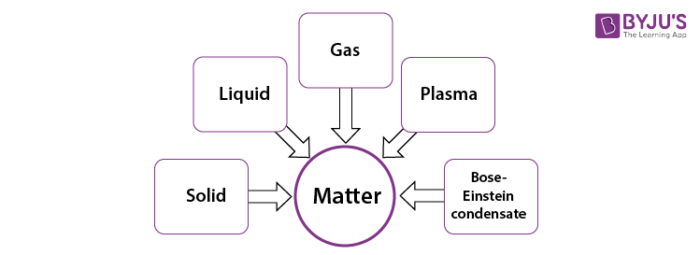
The matter has been characterised into five states by the researchers i.e., solid, liquid, gas, plasma and Bose-Einstein Condensate. Amid these states, the most familiar states of matter that exist around us are solids, liquids and gases.
1. Solid State
When the particles are packed together firmly, they form solids. In solids, the particles only vibrate about their fixed positions, since their kinetic energy is low and not sufficient to let them breakdown away from their common force of the pull. Thus, solids have definite forms and volumes and are not compressible. That’s why they do not flow or diffuse.
Exceptions
- A rubber band can be strained under force, and it recovers to the same shape when the force is removed.
- If a similar rubber band is stretched maximum with excessive force, it breaks.
- The sponge is one more example of a solid, which has minuscule holes in which the air is confined.
- When it is squeezed with a hand, the air is ejected out, and it gets compressed.
2. Liquid State
In liquids, the kinetic energies of the atoms are more than in solids, and the atoms are not fixed to any positions. They move about at will, arbitrarily, all through the liquid. Though, they do not have sufficient kinetic energy to break out of the borderlines of the liquid form. That clarifies why liquids do not have fixed shapes and pour or diffuse at will, but they do have fixed volumes.
Also, when equated to solids, there are more spaces amongst the atoms of liquids, but not sufficient to make liquids compressible.
3. Gaseous State
In gases, the atoms are not crammed together at all, as their kinetic energies are high enough to let them break free from any boundaries. They are unrestricted to move about in arbitrary motion. That is why gases have no fixed figure or volume, and they flow and diffuse readily. They crash into each other, and off the walls of their container. That’s how a gas applies pressure on its vessel. Also, as the spaces between the atoms are large, gases are exceedingly compressible.
4. Plasma State
The fourth state of matter is Plasma. Plasma is comparable to the gaseous state. The state involves super active and super energized atoms in the form of ionized gases. Plasma is created by heating a gas until it loses all its electrons. It exists in stars. The plasma is formed in the sun and stars because of the very high temperatures. The sun and stars radiate because of the existence of plasma in them.
The fluorescent tube and neon sign bulbs contain plasma. The gas present inside these bulbs and tubes is an inert gas. When electricity is passed through them, the gas gets ionized and charged. This charging up creates a glowing plasma, having a particular colour depending on the nature of the gas.
Bose-Einstein Condensates
The model of BEC was thought of by the Indian physicist Satyendra Nath Bose in 1920 and was advanced by the theory of BEC. Later Albert Einstein prophesied a new state of matter – the Bose-Einstein Condensate (BEC).
The BEC is created by freezing a gas of tremendously low density. Bose-Einstein condensate refers to the breakdown of atoms into a single quantum state. It is found at low temperatures when particles are not incapable of moving.
BYJU’S provides detailed information on this topic. To learn more register now!
Access NCERT Solutions for Class 9 Science Chapter 1 here.


thank you
Thank you for the Information .