What is Ammonium Chloride?
NH4Cl is an inorganic compound with the chemical name Ammonium Chloride. It is also known as sal ammoniac, the salt of ammonia and hydrogen chloride.
Ammonium chloride is a by-product of sodium carbonate. Ammonium chloride has diuretic and expectorant effects. In its pure form, it is crystalline salt, white. This compound is highly water-soluble and mildly acidic. Ammonium chloride is used in veterinary medicine for the prevention of urinary stones in sheep, goats, and cattle. When ammonium sulfate and NaCl solutions react, NH4Cl is produced. When a 5% solution of ammonium chloride (by weight) is mixed with water, the resulting solution has a pH value ranging from 4.6 to 6.0.
Table of Contents
- Structure of Ammonium Chloride
- Properties of Ammonium Chloride
- Preparation of Ammonium Chloride
- Chemical properties of Ammonium chloride
- Uses of Ammonium Chloride
- Health effects of Ammonium Chloride
- Frequently Asked Questions – FAQs
Ammonium chloride is an acidifying salt that may be found in the body and in the urine. Ammonium chloride aids in pH regulation and has a modest diuretic impact. This acid-forming salt also has an expectorant action by irritating the mucous membranes, making it useful for cough relief. Ammonium chloride is a crystalline white substance.
Structure of NH4Cl
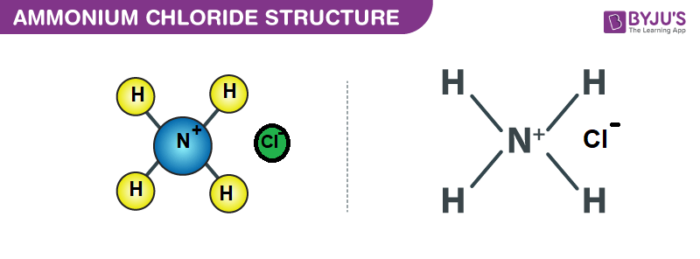
Properties of Ammonium Chloride
| NH4Cl | Ammonium Chloride |
| Molecular Weight/ Molar Mass | 53.491 g/mol |
| Density | 1.53 g/cm³ |
| Boiling Point | 520 °C |
| Melting Point | 338 °C |
Preparation of Ammonium Chloride (NH4Cl)
- Ammonium chloride is prepared commercially by reaction of ammonia (NH3) with hydrogen chloride.
NH3 + HCl → NH4Cl
- Ammonium chloride is also formed as the by-product of the Solvay Process. In this process carbon dioxide and ammonia are passed into a cold saturated solution of sodium chloride.
CO2 + 2 NH3 + 2 NaCl + H2O → 2 NH4Cl + Na2CO3
Chemical Properties of Ammonium Chloride (NH4Cl)
- On decomposition of ammonium Chloride produces ammonia gas and hydrogen chloride.
NH4Cl → NH3 + HCl
- Ammonium chloride also reacts with sodium hydroxide to produce ammonia gas.
NH4Cl + NaOH → NH3 + NaCl + H2O
- Ammonium chloride reacts with sodium carbonate to produce sodium chloride and ammonia gas.
2 NH4Cl + Na2CO3 → 2 NaCl + CO2 + H2O + 2 NH3
Uses of Ammonium Chloride
- It is used in fertilizers as a nitrogen source.
- It is used in medicine (especially in cough medicine) as an expectorant.
- It is used in glue which helps to bond plywood.
- It is used in Leclanche cells in aqueous solutions.
- It is used in food additives – in bread making as a yeast nutrient.
- It is used as an acidifier.
- It is used in cooling baths to create low temperatures.
- They are used as buffer solutions along with ammonia.
- It is given to cattle as feed supplements.
Ammonium Chloride Health Effects
It’s not completely risk-free, as overdosing is a possibility. Higher blood pressure can be caused by ammonium chloride. Irritation, shortness of breath, cough, nausea, and headache are all symptoms of ammonium chloride poisoning. The gases have the potential to cause serious eye discomfort. Chronic exposure can trigger an asthma-like reaction or impair kidney function.
Ammonium chloride is used as a systemic acidifier in the treatment of severe metabolic alkalosis, in the oral acid loading test to identify distal renal tubular acidosis, and in the treatment of several urinary-tract illnesses to keep the urine at an acid pH.
Frequently Asked Questions – FAQs
What are the uses of ammonium chloride?
The primary use of ammonium chloride is as a source of nitrogen in fertilizers (corresponding to 90 per cent of ammonium chloride production worldwide) such as chloro ammonium phosphate. Rice and wheat are the principal crops fertilized in this way. Ammonium chloride is also used as a flux to prepare metals for grinding, galvanizing, or soldering in nickel.
How is ammonium chloride prepared?
Ammonium chloride is produced as one of the products in the Solvay process for the production of sodium carbonate. This compound can also be prepared on an industrial scale by reacting ammonia with hydrochloric acid or gaseous hydrogen chloride. Ammonium chloride is known to occur naturally in certain volcanic areas.
What happens when ammonium chloride is heated?
When heated, ammonium chloride undergoes a decomposition reaction to yield ammonia and gaseous hydrogen chloride as the products. Although this process appears similar to sublimation processes, the change is chemical and not physical.
Is ammonium chloride and bleach dangerous?
If we mix ammonium chloride and bleach it will release chloramine gas. Chloramine gas is toxic in nature, exposure to chloramine gas causes irritation of eyes, nose, throat and lungs.
What is ammonium chloride found in?
Ammonium chloride is found in household products like shampoo, hair colour and bleach, body wash, facial cleanser, conditioner, hand wash, dish wash detergent as well as oil and salt. It’s an important use of electrolytes in dry cell batteries.
Learn more about the structure and the physical and chemical properties of NH4Cl from the experts at BYJU’S.

I am very happy