The Beckmann rearrangement is a process that converts oximes to amides and is used in a variety of industries. In terms of safety and viability, the reaction has improved significantly since its discovery. This paper examines the history of the Beckmann rearrangement and how it has been used to current syntheses of mass-produced, widely available chemicals that formerly required expensive, poisonous, and difficult-to-find reagents.
The Beckmann rearrangement is a process discovered by chemist Ernst Otto Beckmann in the mid-1880s. The reaction transforms oximes to their amides, allowing the nitrogen atom from the C=N bond to be inserted into the carbon chain, establishing a C–N link. It could also make nitriles from aldehydes, depending on the starting material.
|
Definition: The Beckmann rearrangement of ketoximes into amides is an acid-catalyzed process. At higher temperatures, the yield of this process increases. |
Beckmann Rearrangement Chemistry Questions with Solutions
Q1: In rearrangement reactions, what types of isomers are produced?
a) Geometrical isomers
b) Structural isomers
c) Optical isomers
d) Conformational isomers
Answer: b) Structural isomers
Explanation: The atoms in the products have multiple configurations or bonds, but they have the same molecular formula. Because butane and isobutane both have the same number of carbon (C) and hydrogen (H) atoms, their chemical formulae are identical.
Q2: Which chemical rearrangement was the first to be recognised as such by early chemists?
a) Wolff’s rearrangement
b) Pinacole rearrangement
c) Favorskii rearrangement
d) Hofmann rearrangement
Answer: b) Pinacole rearrangement
Explanation: Early chemists identified the pinacol rearrangement as the first molecular rearrangement.
Q3: Which medium is used in the benzylic acid rearrangement reaction?
a) Neutral
b) Strong basic
c) Mild acidic
d) Strong acidic
Answer: b) Strong basic
Explanation: The attack of hydroxide on one of the carbonyl groups initiates the benzylic acid rearrangement process.
Q4: What is a rearrangement reaction with an example?
Answer:
Straight-chain alkanes are transformed into ramified isomers by heating in the presence of a catalyst. Examples include the isomerization of n-butane to isobutane and pentane to isopentane. Highly branched alkanes provide favourable combustion characteristics for internal combustion engines.
Q5: Give the applications of Beckmann Rearrangement.
Answer:
The following are some examples of how this reaction might be used:
- It’s utilised in the pharmaceutical industry to produce paracetamol. The process of converting a ketone to a ketoxime with the help of hydroxylamine achieves this integration.
- It is primarily employed in the production of various steroid and medicinal compounds.
- Some chloro bicyclic lactams can be made via the Beckmann Rearrangement synthesis.
Q6: Give the mechanism of the Beckmann Rearrangement reaction.
Answer:
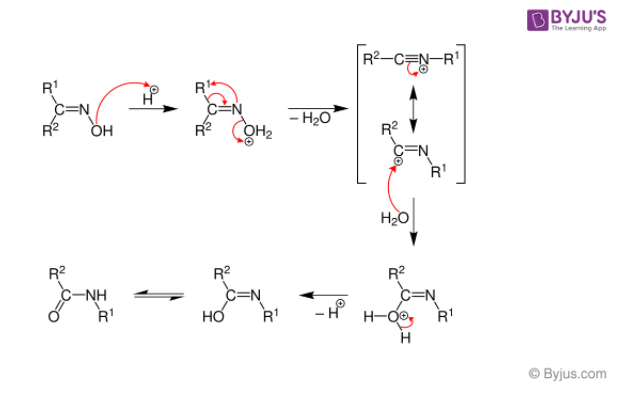
Below is a diagram of the Beckmann Rearrangement process:
- When the hydroxylamine reacts with the cyclohexanone, the oxime is formed.
- After the alkyl substituent “trans” is changed to nitrogen, protonation of the hydroxyl of oxime occurs.
- The N-O link is broken at the same time as the water is released.
- Later, the process of isomerization occurs, which protonates the nitrogen molecule and leads to the creation of amine.
Q7: What is called Cope rearrangement?
Answer:
The Cope rearrangement is a chemical reaction involving the -sigmatropic rearrangement of 1,5-dienes that has been extensively researched. Arthur C. Cope and Elizabeth Hardy designed it. Hepta-1,5-diene is produced by heating 3-methyl-Hexa-1,5-diene to 300°C.
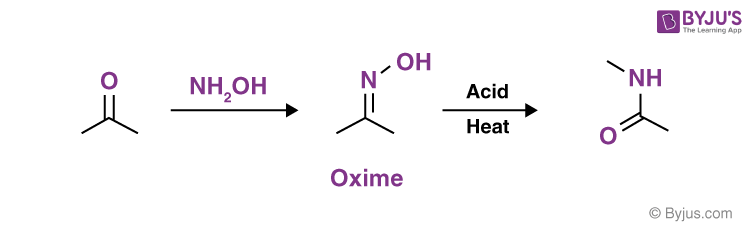
Q8: How is oxime formed?
Answer:
By condensation of an aldehyde or a ketone with hydroxylamine, oximes can be produced. Aldehydes and hydroxylamine are combined to form aldoximes, while ketones and hydroxylamine combine to form ketoximes. In general, oximes are colourless crystals that are difficult to dissolve in liquids.
Q9: What is molecular rearrangement?
Answer:
A rearrangement reaction is an organic reaction in which the carbon skeleton of a molecule is reorganised to produce a structural isomer of the original molecule. A substituent frequently moves from one atom to another inside the same molecule.
Q10: Define Beckmann fragmentation.
Answer:
Beckmann fragmentation is a reaction that competes with Beckmann rearrangement frequently. Fragmentation becomes a feasible reaction route when the group to the oxime is capable of stabilising carbocation formation. The reaction produces a nitrile and a carbocation, which are immediately intercepted and converted into a variety of compounds. Under reaction circumstances, the nitrile can also be hydrolyzed to produce carboxylic acids. Depending on the reaction conditions, fragmentation may be preferred to rearrangement.
Through hyperconjugation, quaternary carbon centres promote fragmentation by supporting carbocation formation. Sulfur can also promote fragmentation, however at a greater distance than oxygen or nitrogen.
Q11: Explain Benzil and Benzilic acid rearrangement.
Answer:
The 1,2-rearrangement of 1,2-diketones to generate -hydroxy–carboxylic acids using a base is known as the benzilic acid rearrangement. The interaction of benzil with potassium hydroxide to create benzilic acid gives this reaction its name. It was the first known example of a rearrangement reaction, achieved by Justus von Liebig in 1838. It’s become a standard reaction in organic synthesis, and it’s been studied extensively. One carbon centre is oxidised while the other is reduced, resulting in an intramolecular redox reaction.
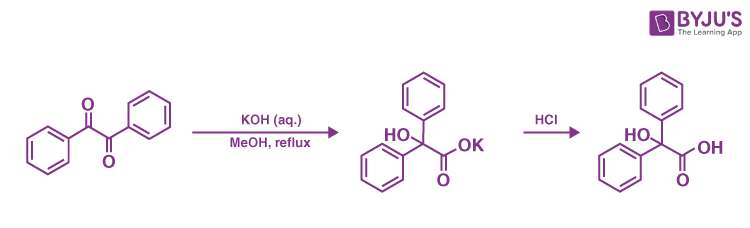
Aromatic, semi-aromatic, aliphatic, and heterocyclic substrates have all been proven to operate in this reaction. When the ketone functional groups have no neighbouring enolizable protons, the process operates optimally because aldol condensation can compete. When employed on cyclic diketones, the reaction is formally a ring contraction. Aryl groups move faster than alkyl groups, according to research, and aryl groups containing electron-withdrawing groups travel the fastest.
Q12: Why does Carbocation rearrangement occur?
Answer:
Carbocation rearrangements are common in organic chemistry, and they are defined by the usage of various structural reorganizational “shifts” inside the molecule as the transfer of a carbocation from an unstable to a more stable form.
Q13: What causes the rearrangement of atoms?
Answer:
Only atoms from the reactants can end up in the products of a chemical reaction. There are no new atoms formed, nor are there any atoms destroyed. Reactants come into contact with each other, links between atoms in the reactants are broken, and atoms rearrange and create new bonds to form products in a chemical reaction.
Q14: What is one of the application of Beckmann transformation?
Answer:
Nylon – 6 is made from caprolactam as a raw ingredient. The Beckmann rearrangement reaction of cyclohexanone and oxime produce caprolactam. Hoechst – Celanese used Beckmann rearrangement to manufacture the drug paracetamol on an industrial scale.
Q15: Explain Beckmann Rearrangement Reaction assisted by Cyanuric Chloride.
Answer:
Using cyanuric chloride and zinc chloride as co-catalysts, the Beckmann rearrangement reaction can be carried out. This sort of Beckmann rearrangement might, for example, create the monomer unit of nylon 12 lactam utilising cyclododecanone as the reactant. This reaction occurs when cyanuric chloride activates the hydroxyl group by an aromatic nucleophilic substitution reaction.
The chemical compound cyanuric chloride has the formula (NCCl)3.
Practise Questions on Beckmann Rearrangement
Q1: What is Benzil used for?
Q2: What type of reaction is Beckmann rearrangement?
Q3: Which group will migrate in Beckmann rearrangement?
Q4: Why do particles rearrange?
Q5: What is the principle of synthesis of benzilic acid from benzil?
Click the PDF to check the answers for Practice Questions.
Download PDF
Read Also:
Comments