A carbocation’s prime job is to stop being a carbocation and there are two approaches to it. It can either get rid of the positive charge or it can gain a negative charge. Both method involves providing the missing electrons to the carbon lacking electrons. It is important to distinguish a carbocation from other kinds of cations.
Table of Content
Carbocation Stability Definition
Stability & Reactivity of Carbocations
Allylic Carbocation Stability
Carbocation Stability Order
A carbocation has a positive charge because it is short of electrons which means the carbon itself is capable of getting another two. This makes it a lewis acid and it also makes a carbocation different from other cations frequently we get to see.
The oxygen atom of H3O+ also has a positive charge but there’s a difference between with carbocation, the H3O+ has a complete octet and the oxygen has a positive charge not because of a shortage of electrons but because it is sharing it with the neighbouring atoms.
Recommended Videos
Stability and Rearrangement of Carbocations

Ring Expansion via Carbonation Rearrangement
Carbocation Stability Definition
To understand why the Markonikov rule will work for carbocation, we need to learn more about the structure and stability of carbocation and the general nature of reactions and also the transition states.
- Carbocations are basically planar in structure and the trivalent carbon is sp2 hybridized. The three substituents are oriented to the corners of an equilateral triangle.
- As there are only six valence electrons on carbon and all of these are in use in sigma bonds the p orbital extending above and below the plane is unoccupied.
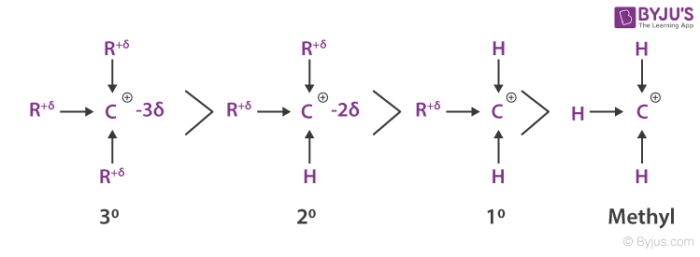
- The carbocation stability is the next important thing we need to understand here and 2 methyl propene might react with H+ to form a carbocation having three alkyl substituents or a tertiary ion of 3o and it might react to form a carbocation having one alkyl substituent with a primary ion of 1o.
- Since the tertiary alkyl chloride is the only product we get to see, the formation of the tertiary cation is evidently favoured over the formation of the primary cation.
- One way of determining carbocation stabilities is to measure the amount of energy to form the carbocation by dissociation of the corresponding alkyl halide, while the tertiary alkyl halide dissociates to give carbocations more easily than secondary or primary ones which results in tri-substituted carbocations are found to be more stable than di-substituted and in turn are more stable than mono-substituted.
- The dissociation enthalpies are much lower in solution because polar solvents can stabilize the ions, but the order of carbocations stability remains the same.
Stability and Reactivity of Carbocations
The stability relationship is fundamental to understanding many aspects of reactivity and especially if it concerns nucleophilic substituents. There are many organic reactions that are widely used in the preparation of desirable organic compounds which include the formation of carbocations.
A very critical step in this reaction is the generation of the tri-coordinated carbocation intermediate. This is completely different from the nucleophilic or electrophilic substitution or electrophilic addition reactions.
- For a mechanism to operate it is very essential that carbocations do not reach a very high energy level as these are inherently high energy species.
- The ionization of 2-chloro-3-methyl propane is endothermic and has 153 Kcal per mol in the gaseous phase.
- A reaction with an activation energy of this magnitude would have a slow rate of reaction at room temperature.
- This is evident that the stability of carbocations greatly increases with solvent and therefore, the results of the gas phase are ignored when determining the reactivity of carbocations are concerned.
- The hydride affinity as a measure of carbocation reactivity is also taken as a common trend in organic chemistry as the results show that the stability of carbocations increases with additional alkyl substituents.
In recent years it has become possible to put the stabilization effect on a quantitative basis. The incorporation of gas-phase measurements determines the proton affinity of alkenes leads to carbocation formation.
R+ + H– → R – H
Allylic Carbocation Stability
Allylic carbocations like allylic radicals have a double bond next to the electron-deficient carbon. The allyl cation is the simplest allylic carbocation. As the allyl cation has only one substituent on the carbon bearing the positive charge it is primarily allylic carbocation.
Allylic carbocation is considered to be more stable than substituted alkyl carbocations because delocalization is associated with the resonance interaction between the positively charged carbon and the adjacent pie (π) bond. The allyl cation can be represented as a hybrid of two equivalent contributing structures.
A distributed charge in a molecule is more stabilizing than a more localized charge and it is also experimentally determined that the double bond of an adjacent vinyl group provides approximately as much stabilization as two alkyl groups hence, the allyl cation 2o isopropyl cation are comparably more stable.
The classification of allylic cations as 1o, 2o, and 3o is determined by the location of the positive charge in the more important contributing structure.
CH2 = CH – CH2+
CH3–CH+–CH3
Carbocation Stability Order
When we compare stabilities of carbocations it must be understood that our standard for each cation is the substrate from which it is formed.
From experimental evidence, we have come to know that 3o carbocation is more stable and need lower activation energy for its formation. Next to this species is the 2o carbocation is more stable than 1o carbocation and requires less activation energy than 1o species.
The 1o and methyl carbocations are so unstable that they are rarely observed in solution. It is also evident that a more stable carbocation intermediate forms faster than a less stable carbocation intermediate species. A system bearing a charge whether positive or negative is considered to be more stable if the charge is delocalized.
The order of stability of carbocation can also be explained by assuming that alkyl groups bonded to a positively charged carbon release electron density toward that carbon and help delocalize the positive charge on the cation. Thereby, electron releasing ability of alkyl groups bonded to a cationic carbon is considered by two effects, inductive effect and the hyper-conjugation.
Methyl cation → ethyl cation → isopropyl cation → tert-butyl cation
The difference in stability between carbocations is much larger than between free radicals. The tert butyl radical is only 12 Kcal more stable than methyl free radical and hence depends upon the substrate with 66 – 72 Kcal more stable than the methyl cation.
Frequently Asked Questions – FAQs
Why does stability of carbocations increase with substitution?
It is very electron-poor for a positively charged species such as a carbocation, and so something that donates electron density to the centre of electron poverty can help stabilize it. It is not correct to suggest, however, that higher substitution carbocations are often more stable than less substituted carbocations.
Are allylic carbocations more stable than tertiary?
While stabilized primary resonance carbocations are less stable than tertiary carbocations (allyl cation, benzyl cation, and methoxymethyl cation), stabilized secondary resonance carbocations are more stable than tertiary carbocations.
Who discovered Hyperconjugation?
The Baker-Nathan influence is presumably recognized among those chemists who obtained their training in physical organic chemistry in the pre-1975 period. The paper would also discuss how Nathan discovered what was considered to be the first instance of hyperconjugation by Baker and his collaborator.
How does conjugation affect stability?
In chemistry, a conjugated structure is a system of bound p orbitals in a molecule with delocalized electrons, which usually decreases the molecule’s total energy and improves stability. It is conventionally depicted as having single and multiple bonds alternating.
What is the stability of Carbanion?
A carbanion is a nucleophile that determines stability and reactivity by several factors: the inductive effect. The voltage can stabilize electronegative atoms adjacent to the charge. The larger the charge-bearing atoms-character, the more stable the anion; the anion ‘s degree of conjugation.
