Carboxylic acid is an organic compound containing a carboxyl group (COOH) attached to an alkyl or aryl group. They react with metals and alkalis to generate carboxylate ions. These reactions of carboxylic acids indicate their acidic nature. The acidity of carboxylic acids is higher in comparison to simple phenols as they react with weak bases like carbonates and bicarbonates to liberate carbon dioxide gas. The carboxylic acids are acidic in nature because the hydrogen in -COOH group can be given forming carboxylate ion.
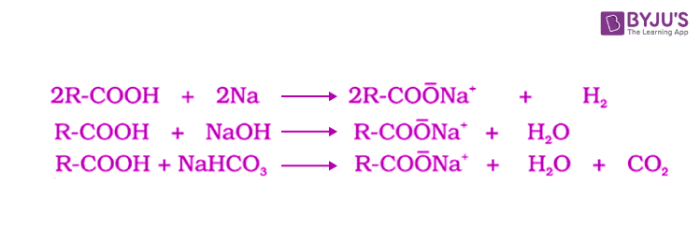
Acidity of carboxylic acids and derivatives
Carboxylic acids dissociate in water to form carboxylate ions and hydronium ions. The carboxylate ion formed is stabilized through resonance by effective delocalization of the negative charge.
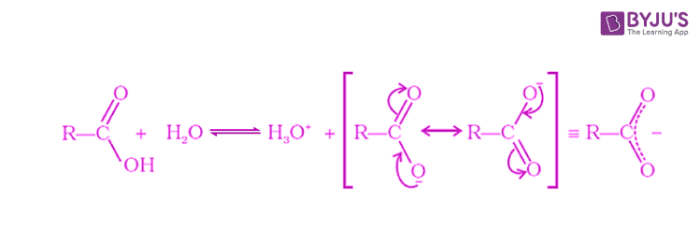
Carboxylic acids are weaker than mineral acids but are strongest among the organic compounds. The acidity of a carboxylic acid is higher than alcohol and even phenols. As discussed above, the carboxylate ion, the conjugate base of a carboxylic acid is stabilized by two equivalent resonance structures in which the negative charge is effectively delocalized between two more electronegative oxygen atoms.
On the other hand in the case of phenols, negative charge is less effectively delocalized over one oxygen atom and less electronegative carbon atoms in phenoxide ion. Therefore, the carboxylate ion exhibits higher stability in comparison to phenoxide ion. Hence, carboxylic acids are more acidic than phenols. Carboxylic acids react with metals and alkalis form carboxylate ions, which only get stabilized due to resonance. The simplest way to understand carboxyl groups is simply by understanding that withdrawal of electrons leads to the increased acidity of carboxyl groups, whereas donation of an electron leads to the decrease of acidity in carboxyl groups.
More Chemistry Articles
The acidity of carboxylic acids further depends on the nature of the substituent alkyl or aryl group attached to the carboxyl group. An electron-withdrawing group ensures effective delocalization of negative charge through resonance or inductive effect. Thus, electron-withdrawing groups increase the stability of the conjugate base formed and hence the acidity of carboxylic acids. On the other hand, electron-donating groups destabilize the conjugate base formed and hence decrease the acidity of carboxylic acids. A general trend can be seen as:
CF3COOH > CCl3COOH > CHCl2COOH > NO2CH2COOH > NC-CH2COOH
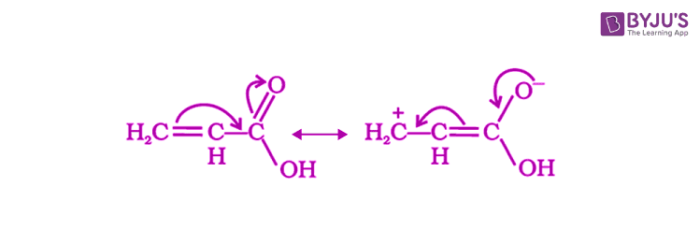
Due to the resonance effect, phenyl or vinyl groups increase the acidity of carboxylic acids which dominates over the decreasing acidity due to the inductive effect.
Recommended Video
Carboxylic Acids Acidity
Frequently Asked Questions – FAQs
What affects the acidity of carboxylic acids?
Which functional group is more acidic?
Why is carboxylic acid stronger than alcohol and phenol?
What happens when sodium reacts with carboxylic acid?
For detailed discussions on the acidity of carboxylic acids, please visit BYJU’S or download the app on your phone, to learn interesting topics on the go!

Explanation was awesome.
Fully NCERT based.
really helpful
#short_notes_