What are Alkenes
Alkenes consist of a large number of loosely held pi bonds. Due to the presence of such bonds they exhibit addition reactions. In addition reaction, the electrophiles (electron seeking species) are added to the double-bonded compounds which result in the formation of the additional product. Under special conditions, in some cases, it has the ability to undergo free radical substitution reactions also. Ozonolysis reaction and oxidation reaction are also prominently exhibited it.
Reactions of alkenes with different compounds
Addition of dihydrogen
Alkanes are formed on addition of one molecule of dihydrogen to alkenes in the presence of different types of catalysts such as palladium, nickel or platinum.
Addition of halogens
Vicinal dihalides are the products formed when alkenes react with halogens such as bromine, chlorine. Iodine is an exception as it does not exhibit addition reaction under normal conditions. Bromine solution loses its reddish-orange colour in carbon tetrachloride solution due to the addition of bromine to an unsaturated site. Hence this reaction is used as a test for the presence of unsaturation in a solution. The reaction of alkenes with halogens is an example of the electrophilic addition reaction.

Addition of hydrogen halides
Alkyl halides are formed when hydrogen halides react with it. The reactivity exhibited by HI is more than HBr and the reactivity of HBr is more than HCl. The addition reaction is possible to both symmetrical and unsymmetrical alkenes. Addition reaction of hydrogen halides to unsymmetrical alkenes takes place in accordance with the following rules:
- Markovnikov rule
- Anti-Markovnikov rule
As per Markovnikov rule when unsymmetric alkene is added to hydrogen halide, halogen gets attached to the carbon having less number of hydrogen.
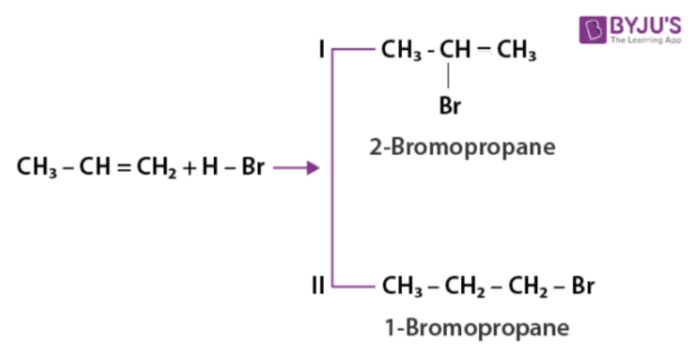
As per anti-Markovnikov rule unsymmetric alkenes add hydrogen halide in the presence of peroxide.The reverse of Markovnikov rule occurs which results in the attachment of halogen to a carbon containing more number of hydrogens.
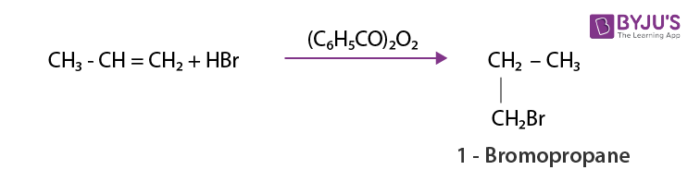
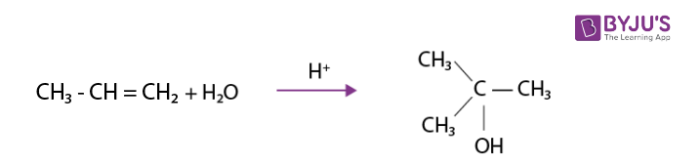
Addition of water
It is made to react with water in the presence of few drops of sulphuric acid.This results in the formation of alcohols as per Markovnikov rule.
To learn more about this topic and other related topics, such as the electrophilic addition reactions of alkenes, register with BYJU’S now!
Oxidation of Alkenes – Video Lesson


can you explain the chemical properties of alkenes
Alkanes are non-polar solvents. Since only C and H atoms are present, alkanes are nonpolar. Alkanes are immiscible in water but freely miscible in other non-polar solvents. Alkanes consisting of weak dipole dipole bonds can not break the strong hydrogen bond between water molecules, hence it is not miscible in water. Click here to learn more about alkenes.