Aim:
To verify the law of conservation of mass in a chemical reaction.
Theory:
- In a chemical reaction, matter is neither created nor destroyed.
- The law of mass conservation states: Mass cannot be created or destroyed in a chemical reaction (except in nuclear reactions, where matter can be converted to energy).
- To make the product, the reactants —> atoms of one or more substances are purely rearranged.
- Total mass of reactants before reaction = Total mass of the product after reaction. Antoine Lavoisier proposed this law.
- Chemicals go through three types of transformations: physical, chemical, and nuclear.
- Physical change: Matter only undergoes phase transitions from solid to liquid and liquid to gas.
- Chemical change: Reactant atoms rearrange to produce new molecules.
\(\begin{array}{l}CaCO_{3} \xrightarrow[]{Heat} CaO + CO_{2}\end{array} \)
- Nuclear change: An atom’s nucleus can be modified by increasing or decreasing the number of protons in it, or by splitting the nucleus. Uranium, for example, can fission to produce Barium and Krypton.
- When reactants react chemically, heat may be produced or released, gas may be released, bubbles may develop, colour may change, or precipitate may form.
| Also Read: Verification of the Law of Conservation of Mass in a Chemical Reaction Viva Questions |
Materials Required:
Two watch glasses, beakers, weighing balance and glass rod.
Chemicals Required:
Distilled water and one of the following sets of chemicals.
Set I → Copper sulphate and Sodium carbonate
Set II → Barium chloride and Sodium sulphate
Set III → Lead nitrate and Sodium chloride
(X)(Y)
Method A
Procedure:
- Take two watch glasses and weigh them on the balancing scale.
- In a watch glass of known mass, weigh 3.6g of BaCl2.2H2O.
- Pour 50 mL of distilled water into a 100 mL beaker. Beaker ‘A’ should be labelled.

- In beaker ‘A,’ dissolve the weighed BaCl2.2H2O.
- Now weigh 8.05g of Na2SO4.H2O in a known-mass watch glass and dissolve it in the other beaker holding 50 ml of distilled water. Label the beaker as ‘B.’
- Take a second beaker. Weigh it and mark it with the letter ‘C.’
- In beaker C, combine the contents of beakers A and B. With a glass rod, stir it.
- The synthesis of (BaSO4) Barium Sulphate results in the formation of a white precipitate in beaker ‘C.’
- Weigh beaker ‘C’ again with the product you obtained and keep track of your findings.
- Subtract the mass of beaker ‘C’ before and after adding the solutions from ‘A’ and ‘B’ beakers to get the mass of the finished product.
Observations:
- Mass of Reactants = 44 g
- Mass of Products = 44 g
- Mass of BaCl2.2H2O = 3.6 g
- Mass of BaCl2, solution = 53.6 g
- Mass of Na2SO4.10H2O = 8.05 g
- Mass of Na2SO4 solution = 58.05 g
- Total mass of reactants is 53.6 g + 58.05 g = 111.65 g (BaCl2, soln.) (Na2SO4 solution.)
- Mass of beaker ‘C’ is C1 = 500 g
- Mass of reaction mixture on adding in beaker ‘C’ is C2 = 611.65 g
- Mass of the product formed = C2 – C1 = 111.65 g
Note: Mass of 50 ml of distilled water = 50.0 g
(density of water = 1 g/mL)
Result:
Thus, Initial mass of reactant = Final mass of the product (reactant mixture).
\(\begin{array}{l}BaCl_{2}(aq) + Na_{2}SO_{4}(aq) \to BaSO_{4}(s) + 2NaCl(aq)\end{array} \)
Method B
Procedure:
- Make a 5% solution of any of the X and Y compounds listed.
- In a conical flask, place a small amount of Y solution and a small amount of X solution.
- As illustrated in the diagram, carefully suspend the ignite tube in the flask. The test tube solution must not flow into the flask.
- Cover the flask with a cork.
- Carefully weigh the flask and its contents.
- Now gently tilt and swirl the flask to combine the solutions X and Y.
- Re-weigh the conical flask.
- Make a note of your findings.
- Note the mass of the conical flask before reaction.
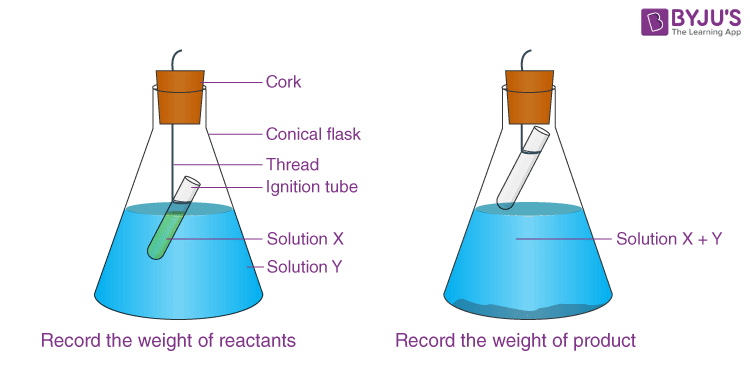
Observations:
- Initial weight of conical flask + ignition tube with X and Y solutions =……g
- Weight of conical flask + ignition tube when solution X and Y are mixed and reaction has taken place =……. g.
- The mass of the reactants equals the mass of the products.
Result:
- Mass of reactants equals mass of products
- In a chemical reaction, there is no mass loss.
Precautions:
- When using the weighing machine, use caution.
- Only use distilled water to make the solution.
- Do not taste any chemical.
- When solution X and Y have been combined, place a cork in the bottle.
- To calculate the resultant mass of the product, subtract the mass of the conical flask + cork.
- When the chemicals (reactants) are mixed, cork should be used to prevent the gas, or vapours, from escaping. The law is verified only in closed systems.
- While recording the initial mass, be sure the chemical does not run out of the ignition tube.
Chemical Reactions:
- Set I : \(\begin{array}{l}CuSO_{4} + Na_{2}CO_{3} \to Na_{2}SO_{4} + CuCO_{3}\end{array} \)
- Set II : \(\begin{array}{l}BaCl_{2} + Na_{2}SO_{4} \to BaSO_{4} + 2NaCl\end{array} \)
- Set III : \(\begin{array}{l}Pb(NO_{3})_{2} + 2NaCl \to 2NaNO_{3} + PbCl_{2}\end{array} \)
Comments