What is Crystallization?
Crystallization is a technique used for the purification of substances. A separation technique to separate solids from a solution.
Crystallization can be defined as the process through which the atoms/molecules of a substance arrange themselves in a well-defined three-dimensional lattice and consequently, minimize the overall energy of the system. When a substance is subjected to crystallization, its atoms or molecules bind together through well-defined angles.
On adding a solid substance in a liquid and stirring it, the solid dissolves in the fluid. But when added more and more solid to the liquid, a point comes after which no more solid dissolves in the liquid. This point is called a saturation point and the fluid is called a saturation solution.

Crystallization
Recommended Videos
Crystallization Chemistry Experiment

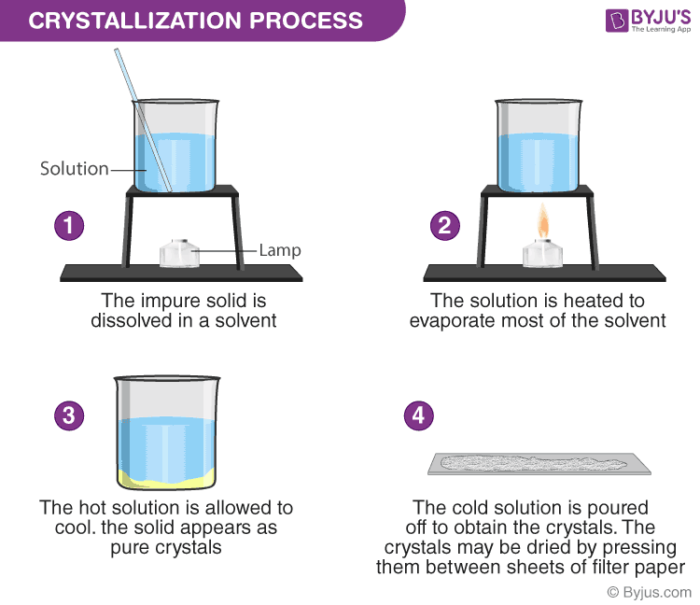
Crystallization Process
- The solution is heated in an open container
- The solvent molecules start evaporating, leaving behind the solutes
- When the solution cools, crystals of solute start accumulating on the surface of the solution
- Crystals are collected and dried as per the product requirement
- The undissolved solids in the liquid are separated by the process of filtration
- The size of crystals formed during this process depends on the cooling rate
- Many tiny crystals are formed if the solution is cooled at a fast rate
- Large crystals are formed at slow cooling rates
Separating Substances via Crystallization
Activity:
Here is an experiment to understand crystallization clearly:
Step 1: Take 50 ml water in a beaker
Step 2: Add sugar in it and stir it
Step 3: Now heat the solution
Step 4: Repeat the process continuously
Step 5: After some time there will be a point at which no more sugar can be dissolved in water. This stage is the saturation point, and the solution is referred to as a saturated solution
Step 6: Now filter the sugar with the help of a filter paper
Step 7: Collect the filtrate in a glass bowl and cool it
Step 8: We will observe that some fine crystals are formed in the bowl
Step 9: The process of filtration can separate these crystals. The liquid left after the removal of crystals is known as mother liquor
Also Read: Crystallization of impure sample
Crystallization Examples
The fixed number of water molecules contained in one formula unit of a salt is known as water of crystallisation. Or, to put it another way, water that is stoichiometrically bonded into crystal. CuSO4 5H2O is the chemical formula for hydrated copper sulphate, for example. Copper sulphate crystallises with 5 molecules of water.
Salt crystallisation is the most practical use of crystallisation, and it is also the most cost-effective technique to create salt today. Compound purification and crystal synthesis are two further uses for the technology. Water of crystallisation may alternatively be defined as the water molecules that make up a crystal’s structure. They form and crystallise the crystals. CuSO4. 5H2O (Copper sulphate) is an antibacterial and antifungal agent that may be used topically.
Application of Crystallization:
- Purification of seawater
- Separation of alum crystals from impure samples
- In the pharmaceutical industry, crystallization is used as a separation and purification process for the synthesis and isolation of co-crystals, pure active pharmaceutical ingredients (API), controlled release pulmonary drug delivery, and separation of chiral isomers.
This was just a brief layout of the process of crystallization. To know about crystallization, other methods of purification of organic compounds and more, register with BYJU’S and download our app.
Frequently Asked Questions – FAQs
What is the definition of the term crystallization?
Crystallization can be defined as the solidification of a liquid substance into a highly structured solid whose atoms or molecules are placed in a well-defined three-dimensional crystal lattice. The smallest individual part of a crystal is called a unit cell. The crystal is made up of millions of such unit cells.
What are the uses of crystallization?
Crystallization is primarily employed as a separation technique in order to obtain pure crystals of a substance from an impure mixture. Another important application of crystallization is its use to obtain pure salt from seawater. Crystallization can also be used to obtain pure alum crystals from an impure alum. In such scenarios, crystallization is known to be more effective than evaporation since it also removes the soluble impurities.
What are the two primary types of crystallization?
Crystallization processes can be broadly categorized into the following two types:
- Evaporative crystallization
- Cooling crystallization
List some examples of crystallization.
Some common examples of crystallization are listed below.
- The crystallization of water to form ice cubes and snow.
- The crystallization of honey when it is placed in a jar and exposed to suitable conditions.
- The formation of stalagmites and stalactites (especially in caves).
- The deposition of gemstone crystals.
What are the advantages of crystallization?
The key advantages of crystallization are listed below.
- A product of high purity can be obtained from one single step via the process of crystallization.
- The dry products formed from crystallization can be directly packaged and stored.
- The energy requirements and the operating temperatures of this process are relatively low.

Very nicely explained.
Nice. 🙂
well explain and good approach of technicalities