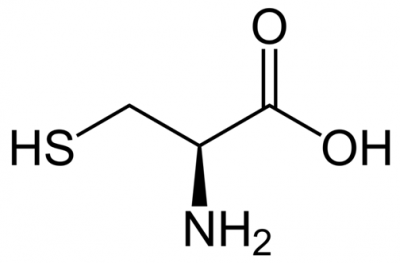
Cysteine is an amino acid present in our body with the chemical formula of HO2CCH(NH2)CH2SH. It is utilised in our bodies as a building block of proteins.
Cysteine can be generally defined as a non-essential amino acid. Foods that have high protein content are usually the ones that contain cysteine. Some major sources are dairy products, poultry products and meat. Some of the plant sources are granola, onions, red peppers, lentils etc.
N-acetyl-L-cysteine (NAC) is the form when ingested as a supplement. The amino acid in context is converted from the above chemical and then converted into glutathione which is a potent antioxidant.
The free radicals present in our bodies are fought by these antioxidants. Radicals are injurious compounds that harm the DNA, cell wall and cell membrane. Many problems have been associated with radicals, such as cancer, breaking down the mucus in the body etc. Cysteine, mainly the L-enantiomer, is a precursor in personal-care, pharmaceutical and food industries.
Source of Cysteine
Cysteine is created from methionine – an essential amino acid by our body. Some of the foods that have the amino acid are enlisted below:
- Pork
- Duck
- Turkey
- Yoghurt
- Ricotta
- Granola
- Chicken
- Oat flakes
- Wheat germ
- Lunch meat
- Sausage meat
- Cottage cheese
Applications of Cysteine
Cysteine also comes into play in the development of flavours.
For instance, during the Maillard reaction, it reacts with sugars to give us meaty flavours. The art of baking uses this nonessential amino acid as a processing aid. Biomolecular structures and their dynamics are explored by the use of this chemical. Other applications of Cysteine are:
- Cigarettes also contain cysteine
- It is also used to treat schizophrenia
- It acts as an antidote for hangovers and liver damage
- Asian countries also use cysteine for personal care applications
- It controls blood sugar levels, and is widely used by patients with Type 2 Diabetes
- Emphysema, cystic fibrosis and asthma have shown great response to this chemical
Discover more about Cysteine, its structure, uses, and other related topics only at BYJU’S Biology


Comments