The organic reactions in which an electrophile replaces an atom connected to an aromatic ring are known as electrophilic aromatic substitution reactions. These reactions involve the replacement of a hydrogen atom from a benzene ring with an electrophile. Aromatic nitrations, aromatic sulphonation, and Friedel-Crafts reactions are a few examples.
|
Definition: When an electrophile replaces the hydrogen atom of benzene, then such reactions are called electrophilic substitution of benzene. |
Electrophilic Substitution of Benzene Chemistry Questions with Solutions
Q-1: The correct order of the electrophilic substitution is
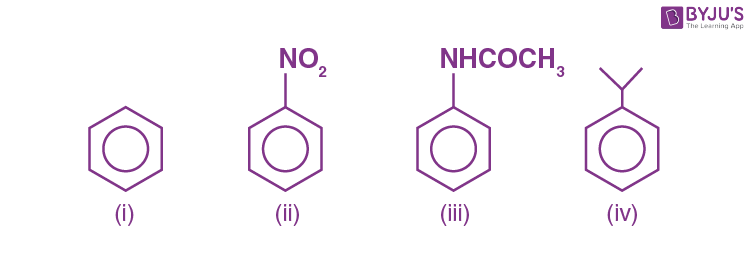
- i>ii>iii>iv
- iii>iv>i>ii
- iii>i>iv>ii
- iii>ii>i>iv
Answer:b) iii>iv>i>ii
Explanation: The rate of electrophilic substitution depends on the nature of the substituent already present in the benzene ring.
If the substituent is o/p directing(activating groups) then the rate of substitution increases. If it is meta directing(deactivating groups) then the rate of substitution decreases.
In the given question, activating groups are -NHCOCH3 and isopropyl groups.The NHCOCH3 is more activating than the isopropyl group as it shows +M effect (l.p donation from N-atom to the ring through resonance increases electron density) making further substitution easy. On the other hand isopropyl just stabilises by +I effect. +M effect is stronger than+I effect. As a result (iii) is more reactive than (iv).
ii) is least reactive towards electrophilic substitution because -NO2 is a deactivating group. Nitro group reduces the electron density in the benzene ring due to its strong–I effect making further substitution difficult, so the rate decreases.
Thus the correct order is iii>iv>i>ii
Q-2: An electrophile is
- Electron hating specie
- Electron loving specie
- Positively charged
- Negatively charged
Answer: b) and c) Electron loving specie and Positively charged
Explanation: The term electrophile is derived from the words “electro” (electron) and “phile” (loving). In short we can say that they are electron loving species.
Any molecule, ion, or atom that is electron-deficient in some way can act as an electrophile. In other words, an electrophile is a reagent that attacks the electron rich system or loves electrons. They are almost always positively charged.
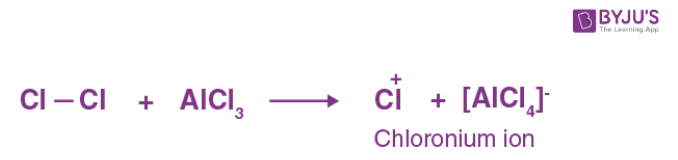
Q-3: What is the role of lewis acid such as AlCl3 in electrophilic substitution reaction?
Answer: Anhydrous AlCl3, as a Lewis acid, aids in the generation of the electrophile.
For example: The electrophile is formed during chlorination as follows:
Q-4: In electrophilic substitution reaction, the arenium ion(intermediate) formed loses it aromaticity because
- It contains sp2 carbon
- A sp3 carbon is formed
- It doesn’t have much resonance
- A positive charge develops
Answer: b) A sp3 carbon is formed
Explanation: Attack of electrophile results in the formation of σ-complex or arenium ion in which one of the carbon is sp3 hybridised as shown below: 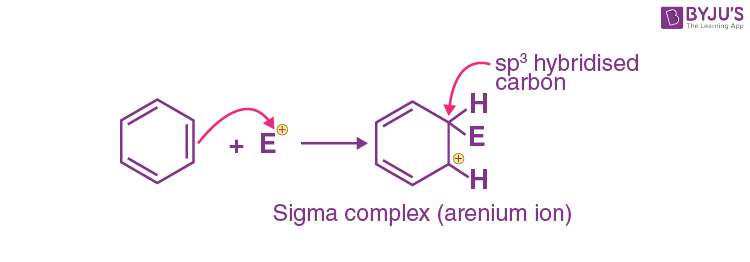
Because electron delocalisation stops at sp3 hybridised carbon as a result the sigma complex or arenium ion loses its aromatic character.
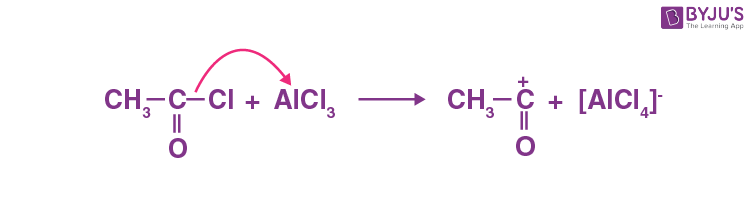
Q-5: In the presence of Lewis acids (AlCl3), the reaction of benzene with ethanoyl chloride yields electrophile. Show the mechanism for the same.
Answer: The generation of electrophile by lewis acid (AlCl3) is shown below:
Q-6: To restore aromatic character, sigma complex releases
- Hydride ion
- Proton
- Electrophile
- Electrons
Answer: b) Proton
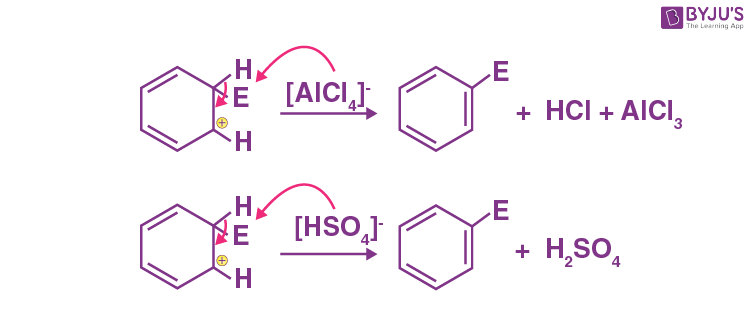
Explanation: To restore the aromatic character, σ -complex releases a proton from sp3 hybridised carbon on attack by [AlCl4]– (in case of halogenation, alkylation and acylation) and [HSO4] – (in case of nitration).
The mechanism is shown below:
Q-7: Among the following, which is most reactive towards electrophilic substitution?
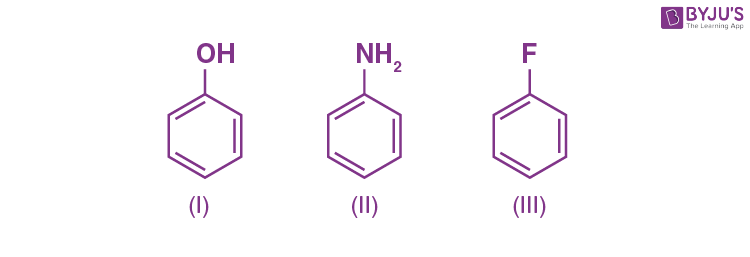
Answer: II
Explanation: NH2 and OH are activating groups, and their +M effect activates rings. The strength of +M decreases as the electronegativity of the donating atom in the group increases. Because N in NH2 is less electronegative than O in OH, NH2 is a better activating group. As a result, the rate of electrophilic substitution in II will increase.
On the other hand, in III) -F( a halogen) is moderately deactivating. Because of its strong – I effect, overall electron density on benzene ring decreases. It makes further substitution difficult.
Q-8: Benzene has a lower electrophilic substitution reaction reactivity than
- Thiophene
- Furan
- Pyrrole
- All of the above
Answer: d) All of the above
Explanation: Five membered heterocycles ( pyrroles, furan and thiophene) are pi-excessive and are characterised by their ability to undergo electrophilic substitution reactions on the ring carbon atoms.The partial positive charge on heteroatom hinders the attack of electrophile, while negatively charged carbon atoms facilitate the attack of electrophile as shown below. This makes them more reactive towards electrophilic substitution reactions than benzene.(it is less electrophilic). 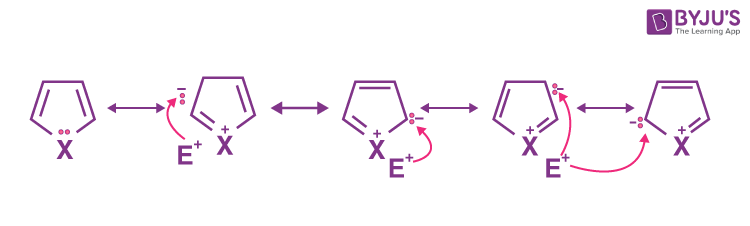
Q-9: Which of the following statements about electrophile is not true?
- Electrophiles are positively or negatively charged species with vacant orbitals.
- The most electron-populated part of a nucleophile attacks electrophiles.
- Carbenes and nitrenes are examples of chemical electrophiles
- Electrophiles are lewis base
Answer: d) Electrophiles are lewis base
Explanation: An electrophile is an electron deficient specie that accepts electrons, therefore acting as a lewis acid.
A lewis base is a specie that donate electron pairs to the electron deficient molecule.
Q-10: Which of the following sets of substituents are all meta directing in electrophilic substitution reaction?
a) Cl,NH2,CH3
b) CN, SO3H,CHO
c) COCH3,CONR2,NH2
d) OCOCH3,OH, CN
Answer: b) CN, SO3H,CHO
Explanation:
meta-directing groups: CN, SO3H, CHO, COCH3, CONR2
O/p-directing groups: Cl, NH2, CH3, OH
Q-11: Which of the following is an intermediate in bromination of toluene?
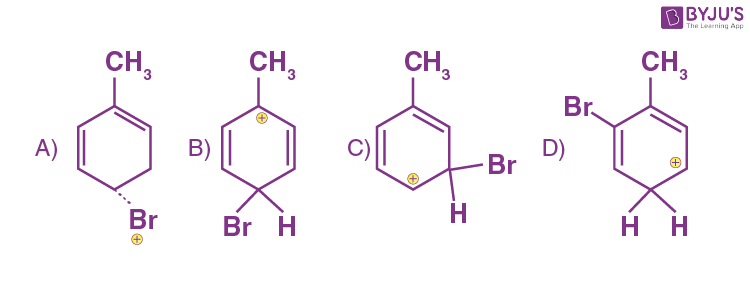
Answer: b)
Explanation: Mechanism of bromination of toluene at para position is shown below: 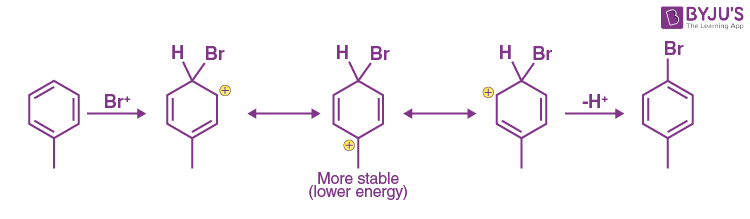
Q-12: What will be the product A?
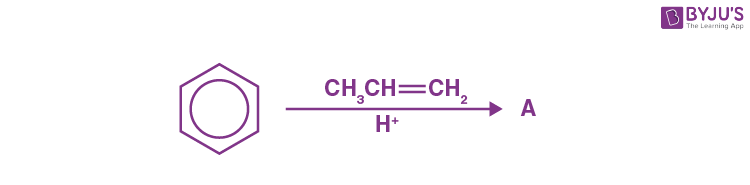
a) 2-phenyl propane
b) Propyl benzene
c) Phenyl propene
d) 1-phenyl-prop-2-ene
Answer: a) 2-phenyl propane
Explanation: 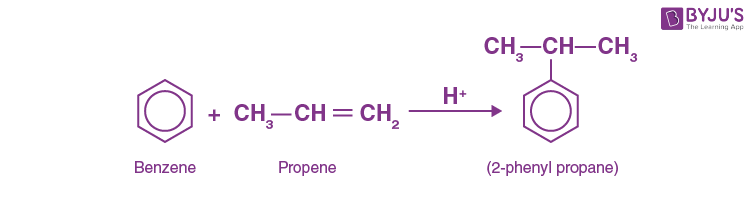
Q-13: The mechanism of electrophilic substitution of benzene includes two steps:
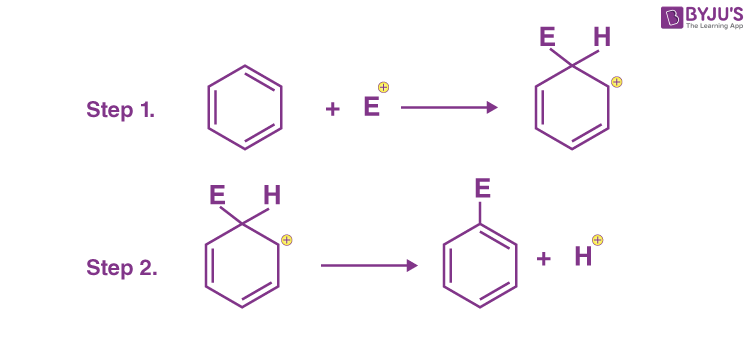
Which of the following statements is correct?
- Both the steps are exothermic
- Both the steps are endothermic
- I step is endothermic and II is exothermic
- I step is exothermic and II is endothermic
Answer: c) I step is endothermic and II is exothermic
Q-14: If the following aromatic compounds undergo bromination ,then
i) The compound that reacts fast
ii) The compound that reacts slowly
Answer: i) B
II) C
Q-15: Which of the following aromatic compounds is alkylated by Friedel craft with propyl chloride in the presence of lewis acid?
- Benzoic acid
- Ethyl benzoate
- Anisole
- Trifluoromethyl Benzene
Answer: c) Anisole
Explanation: In anisole, -OCH3 group is highly activating in nature due to resonance than -COOH,-COOCH2CH3 and CF3 in benzoic acid, ethyl benzoate and trifluoromethyl benzene respectively. Therefore will undergo Friedel craft with propyl chloride more readily.
Practise Questions on Electrophilic Substitution of Benzene
Q-1: Is it possible for an electrophile to be a neutral specie?
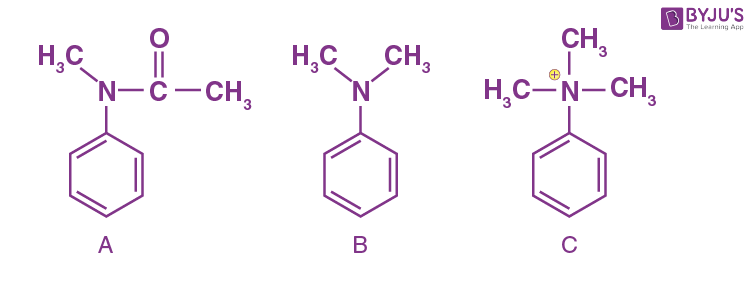
Q-2: For the following aromatic compounds, answer the following questions: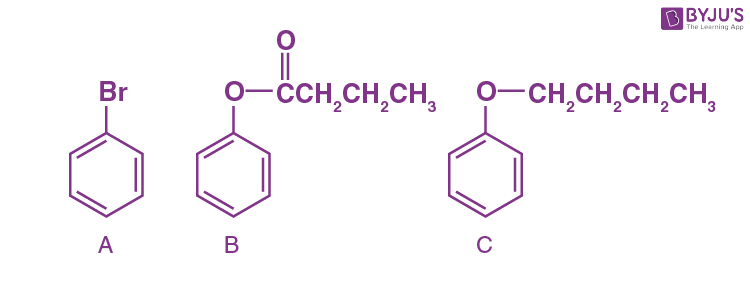
Reaction: Friedel craft reaction
i) Which will show a fast reaction?
ii) Which will show a slow reaction?
Q-3: What is the major product for the reaction of phenyl benzoate with nitrating mixture?
Q-4: Which of the following electrophilic substitution steps is the slowest?
- Removal of hydrogen
- Formation of positive charge by electrophile in arene
- Electron donation to the electrophile via pi bond in arene
- Resonance stabilisation of positive charge through delocalisation
Q-5: Arrange the following in decreasing order of their reactivity towards electrophilic substitution.
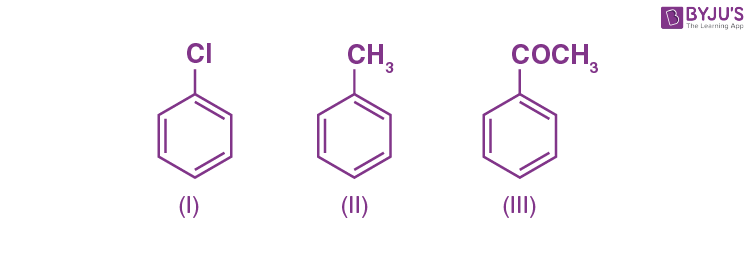
Click the PDF to check the answers for Practice Questions.
Download PDF
Recommended Videos
Electrophilic Substitution Of Benzene