“Fatty acids are a type of lipid composed of carbon, hydrogen, and oxygen arranged as a variable-length linear carbon chain skeleton with an even number of atoms at one end.
There are fatty acids with 2 to 30 carbon atoms or more, but the most common and important ones have 12 to 22 carbon atoms and are found in many different animal and plant fats.:
They are rarely found free in nature and are the primary constituents of:
- Triacylglycerols (or triglycerides);
- Diacylglycerols;
- Monoacylglycerols (the last two families of compounds are often added to processed foods);
- Phospholipids of cell membranes;
- Sterol esters.
Fatty acids are the building blocks of fat in our bodies and the food we consume. During digestion, the body converts fats into fatty acids, which are then absorbed into the bloodstream. Fatty acid molecules are typically joined in groups of three to form a molecule known as a triglyceride. Triglycerides are also produced in our bodies as a result of the carbohydrates we consume.
Fatty acids perform a variety of vital functions in the body, including energy storage. When glucose (a type of sugar) is unavailable for energy, the body turns to fatty acids to power the cells.
Table of Contents
- Classification of Fatty Acids
- List of Fatty Acids
- Production of Fatty Acids
- Properties of Fatty Acids
- Uses of Fatty Acids
- Frequently Asked Questions – FAQs
Classification of Fatty Acids
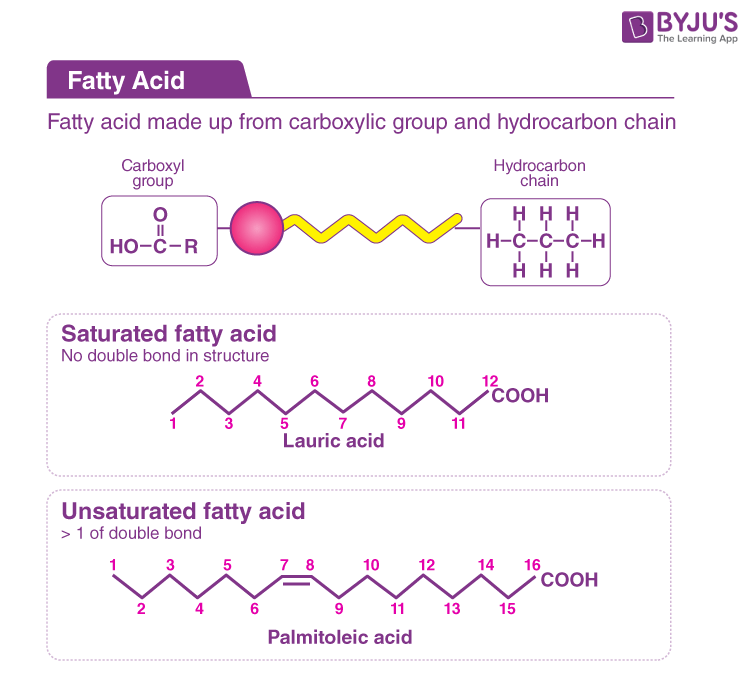
1. They are classified into three types based on their degree of saturation/unsaturation in the carbon chain:
- If there is no double bond, the fatty acid is saturated.
- If there is one double bond, the fatty acid is monounsaturated,
- If there are two or more double bonds, the fatty acid is polyunsaturated.
2. Furthermore, they can be divided into two broad classes based on the presence or absence of double/triple bonds:
- If there are no double bonds in the carbon chain, it is saturated.
- If there are one or more double bonds in the carbon chain, it is unsaturated.

3. They are classified as follows based on their ability or inability to be synthesised by animals, and whose deficiency can be reversed by dietary addition:
- Essential fatty acids
- Not essential
4. They can be functionally classified as follows:
- Short-chain fatty acids: up to 6 carbon atoms
- Medium-chain fatty acids: 8 to 12 carbon atoms.
- Long-chain fatty acids: 14 to 18 carbon atoms
- Very long-chain fatty acids: 20 carbon atoms and up.
5. Other fatty acids
- Oxygenated fatty acids- They have hydroxyl, keto, and epoxy groups; ricinoleic acid, the main fatty acid in castor oil, is an example.
- Cyclic fatty acids- They have a cyclic unit with three, five, or even six carbon atoms, similar to prostaglandins.
List of Fatty Acids
|
Type |
Common Names |
IUPAC Names |
|---|---|---|
|
Saturated fatty acids |
Butyric acid |
Butanoic acid |
|
Caproic acid |
Hexanoic acid |
|
|
Caprylic acid |
Octanoic acid |
|
|
Capric acid |
Decanoic acid |
|
|
Lauric acid |
Dodecanoic acid |
|
|
Myristic acid |
Tetradecanoic acid |
|
|
Palmitic acid |
Hexadecanoic acid |
|
|
Stearic acid |
Octadecanoic acid |
|
|
Arachidic acid |
Icosanoic acid |
|
|
Behenic acid |
Docosanoic acid |
|
|
Lignoceric acid |
Tetracosanoic acid |
|
|
Monounsaturated fatty acids |
Caproleic acid |
Dec-9-enoic acid |
|
Lauroleic acid |
(Z)-dodec-9-enoic acid |
|
|
Myristoleic acid |
(Z)-tetradec-9-enoic acid |
|
|
Palmitoleic acid |
(Z)-hexadec-9-enoic acid |
|
|
Oleic acid |
(Z)-octadec-9-enoic acid |
|
|
Elaidic acid |
(E)-octadec-9-enoic acid |
|
|
Vaccenic acid |
(E)-octadec-11-enoic acid |
|
|
Gadoleic acid |
(Z)-icos-9-enoic acid |
|
|
Erucic acid |
(Z)-docos-13-enoic acid |
|
|
Brassidic acid |
(E)-docos-13-enoic acid |
|
|
Nervonic acid |
(Z)-tetracos-15-enoic acid |
|
|
Polyunsaturated fatty acids |
Linoleic acid |
(9Z,12Z)-octadeca-9,12-dienoic acid |
|
alpha-Linolenic acid |
(9Z,12Z,15Z)-octadeca-9,12,15-trienoic acid |
|
|
gamma-Linolenic acid |
(6Z,9Z,12Z)-octadeca-6,9,12-trienoic acid |
|
|
Columbinic acid |
(5E,9E,12E)-octadeca-5,9,12-trienoic acid |
|
|
Stearidonic acid |
(6Z,9Z,12Z,15Z)-octadeca-6,9,12,15-tetraenoic acid |
|
|
Mead acid |
(5Z,8Z,11Z)-icosa-5,8,11-trienoic acid |
|
|
Dihomo-γ-linolenic acid |
(8Z,11Z,14Z)-icosa-8,11,14-trienoic acid |
|
|
Arachidonic acid |
(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoic acid |
|
|
Eicosapentaenoic acid |
(5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoic acid |
|
|
Docosapentaenoic acid |
(7Z,10Z,13Z,16Z,19Z)-docosa-7,10,13,16,19-pentaenoic acid |
|
|
Docosahexaenoic acid |
(4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-h |
Production of Fatty Acids
Industrial
- Fatty acids are typically produced industrially by hydrolyzing triglycerides and removing the glycerol. Another source is phospholipids. Some fatty acids are synthesised through the hydrocarboxylation of alkenes.
- Specific industrial processes for topical skin creams produce hyper-oxygenated fatty acids. The method works by introducing or saturating peroxides into fatty acid esters in the presence of ultraviolet light and gaseous oxygen bubbling at controlled temperatures.
In Animals
During lactation, fatty acids are primarily formed from carbohydrates in the liver, adipose tissue, and the mammary glands of animals.
Properties of Fatty Acids
- Fatty acids undergo the same reactions as other carboxylic acids, such as esterification and acid-base reactions.
- The acidities of fatty acids do not vary greatly, as indicated by their pKa values.
- The solubility of fatty acids in water decreases as chain length increases so that longer-chain fatty acids have little effect on the pH of an aqueous solution.
- Fatty acids exist at their conjugate bases, such as oleate, near neutral pH.
- Auto-oxidation occurs when unsaturated fatty acids undergo a chemical change. The presence of trace metals speeds up the process, which requires oxygen (air).
- Ozonolysis- Ozone is capable of degrading unsaturated fatty acids. This reaction is used in the synthesis of azelaic acid from oleic acid.
Uses of Fatty Acids
Fatty acids have numerous commercial applications.
- They are used in the production of many food products.
- In the production of soaps, detergents, and cosmetics.
- Soaps are fatty acid sodium and potassium salts. Some skin-care products contain fatty acids, which can help maintain the appearance and function of healthy skin.
- Dietary supplements containing fatty acids, particularly omega-3 fatty acids, are also widely available.
- Fatty acids are also converted to fatty alcohols and fatty amines via their methyl esters, which are factors in the development of surfactants, detergents, and lubricants.
- Emulsifiers, texturizing agents, wetting agents, anti-foam agents, and stabilising agents are all examples of fatty acids.
Frequently Asked Questions on Fatty Acid Groups
What types of groups do fatty acids belong to?
A fatty acid is composed of a straight chain of an even number of carbon atoms, with hydrogen atoms along the length of the chain and at one end, and a carboxyl group (COOH) at the other end. It is the carboxyl group that causes it to be an acid (carboxylic acid).
What are the functions of fatty acids in our bodies?
Fatty acids are the building blocks of fat in our bodies and the food we consume. During digestion, the body converts fats into fatty acids, which are then absorbed into the bloodstream. Fatty acid molecules are typically joined in groups of three to form a molecule known as a triglyceride.
What are the three primary types of fatty acids?
Fatty acids are classified into three types: saturated, monounsaturated, and polyunsaturated.
Is there an amino group in fatty acids?
An amino acid is a simple organic compound that contains both a carboxyl (—COOH) and an amino (—NH2) group, whereas a fatty acid is a carboxylic acid that contains a hydrocarbon chain and a terminal carboxyl group, particularly any of those found as esters in fats and oils.
What are some fatty acid examples?
Fats, oils, cholesterol, and steroids are a few examples.
Comments