Fractional distillation is used to separate a mixture of two or more miscible liquids with a difference in boiling points of less than 25 K.For example, the distillation process is utilised for
separation of various gases from air, various divisions of petroleum products etc. Repeated distillations and condensations are used in the process, and the mixture is usually divided into component parts.
|
Definition: Fractional distillation is a type of distillation in which miscible liquids are separated from each other based on their boiling points. |
Fractional Distillation Chemistry Questions with Solutions
Q-1: A simple fractional column tube is packed with
- Metal beads
- Glass beads
- Plastic beads
- Wooden beads
Answer: b) Glass beads
Explanation: A simple fractionating column is a glass-bead-filled tube. The beads provide a surface for the vapours to repeatedly cool and condense.
Q-2: Due to the presence of_____________, fractional distillation differs from simple distillation.
- Water outlet
- Condenser
- Thermometer
- Fractionating column
Answer: d) Fractionating column
Explanation: The existence of a fractionating column distinguishes fractional distillation from distillation. A fractionating column in a laboratory is a piece of glassware used to separate vaporised mixtures of liquid molecules with similar volatility.
Q-3: Fractional distillation cannot separate which of the following?
- Ethanol + water
- Crude oil
- Separation of different gases from air
- Acetone + water
Answer: d) Acetone + water
Explanation: The boiling point of water is 100o Celsius, whereas the boiling point of acetone is 56o Celsius, a discrepancy of 44o Celsius. As a result, the separation method is chosen in which the difference in their boiling points is more significant than 25K. That is the process of distillation.
Q-4: What is a theoretical plate?
Answer: Fractionation is the same as a series of distillations, in which the separation is accomplished through a series of distillations or repeated vaporisation-condensation cycles. Each vaporisation-condensation cycle produces an equilibrium stage, also called a theoretical stage or plate. For efficient fractionation and separation of the vapour or liquid combination, a number of such theoretical stages may be necessary.
Q-5: Which graphical method is used to determine the number of theoretical stages required?
- Lineweaver Burk plot
- Eadie hofstee plot
- Michaelis menten plot
- McCabe Thiele plot
Answer: d) McCabeThiele plot
Q-6: Match the column I with column II
|
Column I |
Column II |
|
A) To separate liquids that decompose at temperatures lower than their boiling points. |
i) Simple distillation |
|
B) To remove non-volatile contaminants from liquid. |
ii) Fractional Distillation |
|
C) To separate two or more liquids with almost identical boiling points. |
iii)Vacuum Distillation |
|
D) To remove non-volatile contaminants from steam volatile liquids that are insoluble in water. |
iv) Steam Distillation |
Answer: A-iii),B-i), C-ii), D-iv)
Q-7: Fill in the blanks
a) In the fractional distillation procedure, _______ is very crucial.
b) Fractional distillation can be used to remove______ from rainwater and subsurface water.
c) The fractional distillation of crude oil produces _________ which is a combination of different compounds.
d) The components of mixture have different _______ points in fractional distillation.
e) In the separation of crude oil,___________ is obtained which is used in polishes, candles and electric insulators.
Answers:
- Heat
- Contaminants
- Gasoline
- Boiling points
- Paraffin wax
Q-8: Select the incorrect statement from the following options:
a) For fractionation columns to execute and operate well, the diameter of the column must be properly sized.
b) Various designs and sizes are available for fractionating columns.
c) Fractional distillation is the process of separation of ethanol from water.
d) There is just one fixed standard for fractionating columns.
Answer: d) There is just one fixed standard for fractionating columns.
Q-9: Methyl alcohol and acetone can be separated by fractional distillation. The boiling points of methyl alcohol and acetone are _____ and _______ respectively.
- 337.7 K and 329 K
- 329 K and 337.7 K
- 350 K and 438 K
- 438 K and 350 K
Answer: a) 337.7 K and 329 K
Q-10: What are the different constraints of fractional distillation?
Answer: The major limitations of fractional distillation are:
- It is costly because fractional distillation necessitates immense structures, heavy-duty materials, and sophisticated apparatus.
- Fractional distillation, like any other heat-based process, poses a variety of hazards to those who work with it. One of the most serious risks is explosion, which might occur if the system is not equipped with the necessary safeguards.
- Fractional distillation is not environmentally detrimental in and of itself; the environmental impact is determined by the sorts of mixtures distilled. When crude oil is refined, large volumes of hazardous compounds are released into the atmosphere. It can also contribute to the contamination of rivers, streams, and other bodies of water, which occurs when refineries dump wastewater inappropriately.
Q-11: Draw the fundamental setup for fractional distillation.
Answer: The setup for fractional distillation is shown below: 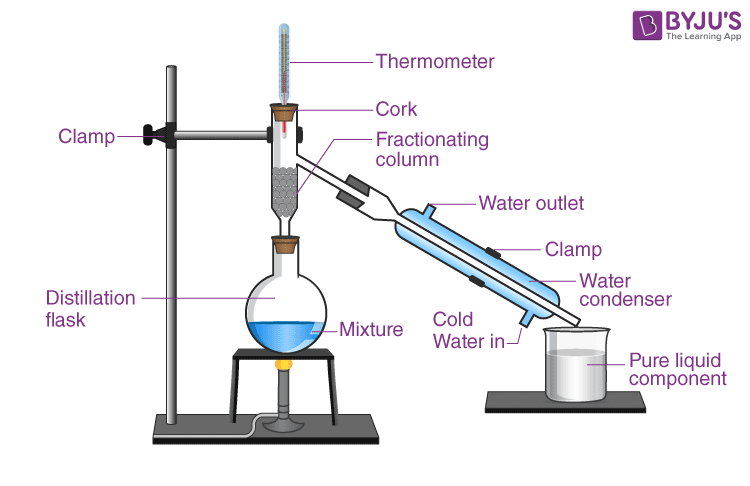
Q-12: In fractional distillation, the gas with ________ boiling point is cooled first.
- High
- Low
- Very low
- Can’t predict
Answer: a) High
Explanation: Gas that has the greatest boiling point is the easiest to compress hence it will condense into liquid first.
Q-13: Chloroform and aniline can be separated by fractional distillation. State whether the statement is true or false.
Answer: False
Explanation: Aniline has a boiling point of 184 degrees Celsius, while chloroform has a boiling point of 61 degrees Celsius. Because the boiling points of the two miscible liquids give a large difference, fractional distillation cannot separate them.
Q-14: Is the fractional distillation able to completely separate the mixtures?
Answer: No, this procedure isn’t completely efficient. Though the components will be separated, there may be a small amount of other components present.
Q-15: What is the purpose of oil bath in fractional distillation?
Answer: An oil bath is a heated bath that is widely used in laboratories to heat up chemical reactions. It consists of an oil container heated by a hot plate or a Bunsen burner.
To avoid direct heating of the reaction mixture during fractional distillation, we keep it in an oil bath. As a result, an oil bath is required to control the temperature of the mixture that needs to be separated.
Practise Questions on Fractional Distillation
Q-1: Which of the gases listed below cannot be separated from air using fractional distillation?
- Helium
- Argon
- Oxygen
- Nitrogen
Q-2: What are the requirements for fractional distillation to separate the mixture?
Q-3: Below are boiling points of some liquids
|
Liquid |
Boiling point (o Celsius) |
|
Water |
100 |
|
n-propanol |
98 |
|
Isopropyl alcohol |
82 |
|
Propionaldehyde |
48.8 |
Which distillation is appropriate for the following mixture, if only one distillation type is allowed?
Mixture: water + Isopropyl alcohol
Q-4: What is the benefit of the long fractionating column?
Q-5: Fractional distillation is employed for
- Heterogeneous solid mixtures
- Homogeneous mixture of solid and liquid
- Homogeneous fluid mixtures
- Heterogeneous fluid mixtures
Click the PDF to check the answers for Practice Questions.
Download PDF
Recommended Videos
How is Crude Oil Processed?

Types of Distillation

Comments