What is the Fries Rearrangement Reaction?
- Fries Rearrangement is an organic rearrangement reaction in which an aryl ester is transformed into a hydroxy aryl ketone with the help of a Lewis acid catalyst and an aqueous acid.
- In this reaction, an acyl group belonging to the phenolic ester migrates to the aryl ring.
- It is important to note that Fries rearrangement is ortho and para selective, i.e. the acyl group attaches itself at the ortho or para positions of the aryl ring.
- The selectivity of the reaction can be directed by modifying the reaction conditions (such as the temperature under which the reaction is conducted, or the solvent used in the reaction).
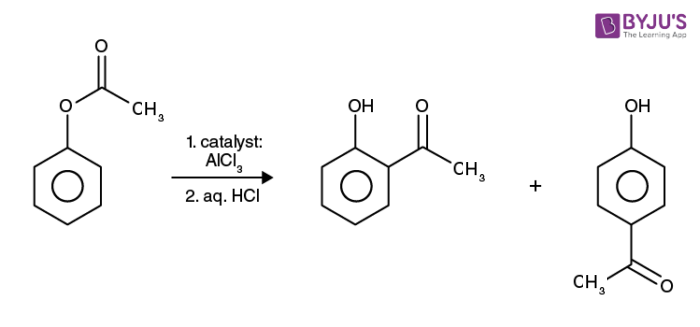
An illustration detailing the Fries rearrangement undergone by phenyl acetate (acetoxy benzene) is provided above. Note that the products feature ortho and para migrations of the acyl group.
Fries Rearrangement Mechanism
Initially, the carbonyl oxygen belonging to the acyl group forms a complex with the Lewis acid catalyst (usually AlCl3). The formation of the complex with the carbonyl oxygen is favoured over the complexation of the phenolic oxygen since the carbonyl oxygen is richer in electrons and is, therefore, a better Lewis base.
Now, the bond between the phenolic oxygen and the acyl complex becomes polarised, resulting in the rearrangement of the AlCl3 bond to the phenolic oxygen. This results in the generation of an acylium carbocation.
The acylium carbocation goes on to attack the aromatic ring via an electrophilic aromatic substitution reaction. It is important to note that the orientation of this electrophilic aromatic substitution is temperature-dependent. Low reaction temperatures favour substitutions at the para position and relatively high temperatures favour ortho substitution.
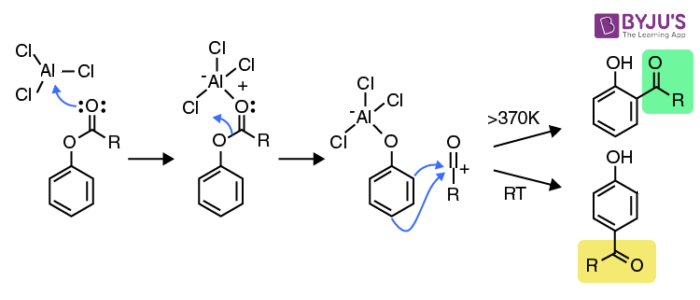
The mechanism of the Fries rearrangement reaction is illustrated above. The use of a non-polar solvent in this reaction also favours the formation of ortho-substituted products. Highly polar solvents favour para substitution in this reaction.
Limitations of Fries Rearrangement
The key limitations of Fries rearrangement are listed below.
- Owing to its relatively harsh reaction conditions, only esters with relatively stable acyl components can be used in this reaction.
- Low yields are obtained when heavily substituted acyl components exist.
- The presence of deactivating or meta-directing groups on the aromatic ring results in low yields.
To learn more about Fries rearrangement and other important named reactions in organic chemistry (such as the Friedel-Crafts acylation reaction), register with BYJU’S and download the mobile application on your smartphone.

why fries rearrangement is selective at ortho and para positions?
what makes it selective?
The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries. The reaction is ortho,para-selective so that, for example, the site of acylation can be regulated by the choice of temperature. Only sterically unhindered arenes are suitable substrates, since substituents will interfere with this reaction.