Gattermann Koch’s reaction mechanism begins with the formation of the reactive species with the help of the acid. The overall aim of the reaction is to attach a formyl group (-CHO group) to an aromatic system. An example of the Gattermann – Koch reaction is given below.
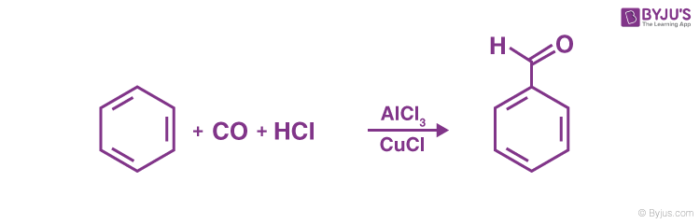
The Gattermann – Koch reaction is not applicable to phenol and phenol ether substrates. If zinc chloride is used as a catalyst in the Gattermann – Koch reaction, traces of copper(I) chloride is often necessary since it acts as a co-catalyst.
Table of Contents
- Gattermann – Koch Reaction Mechanism
Gattermann – Koch Reaction Mechanism
Step 1
The first step of the Gattermann Koch reaction mechanism is the generation of the reactive species which can later be used to react on the aromatic ring. Since carbon monoxide acts as a lewis base, it can accept a proton from the hydrochloric acid. This results in a positively charged molecule which has different resonance structures. One such resonance structure displays a positive charge on the carbon, explaining the reactivity of the hybrid. This species can act as an electrophile while reacting with the aromatic ring. However, it is more likely to be the target of a nucleophilic attack from the chloride ion in the hydrochloric acid.

Step 2
When a Lewis acid (aluminium chloride) is added, it easily removes a chloride ion from the species. The species now reverts to the reactive formyl cation.

Step 3
An electrophilic aromatic substitution occurs at the aromatic ring. The aromatic ring acts as a nucleophile and donates an electron pair to the formyl cation. The temporary loss of aromaticity is quickly solved by the expulsion of a proton.

Thus, the formyl group is attached to the aromatic ring via the Gatterman – Koch reaction. In the example shown in the above mechanism, benzaldehyde is formed from the treatment of benzene with carbon monoxide and hydrochloric acid in the presence of aluminium chloride.

Comments