What is Hexane?
Hexane is an unbranched hydrocarbon with the formula C6H14.
- Hexane is a nonpolar molecule with a weak intermolecular interactions occur between the molecules of pure liquid hydrocarbons.
- It is a highly volatile, flammable toxic chemical which is a by-product made from crude oil.
- Hexane isomers are to a great extent lifeless, and are every now and again utilized as an organic solvent since they are very non-polar
Other names – Amyl Carbinol, 1-Hexanol, Hexyl alcohol, 1-Hydroxyhexane
| C6H14 | Hexane |
| Density | 655 kg/m³ |
| Molecular weight/ Molar mass | 86.18 g/mol |
| Boiling point | 68.5 to 69.1 °C |
| Melting point | −96 to −94 °C |
| Chemical formula | CH3(CH2)4CH3 |
Table of Contents
- Hexane Structure-C6H14
- Physical Properties of Hexane-C6H14
- Chemical Properties of Hexane-C6H14
- Uses of Hexane-C6H14
- Health Hazards
- Frequently Asked Questions
Hexane Structure – C6H14
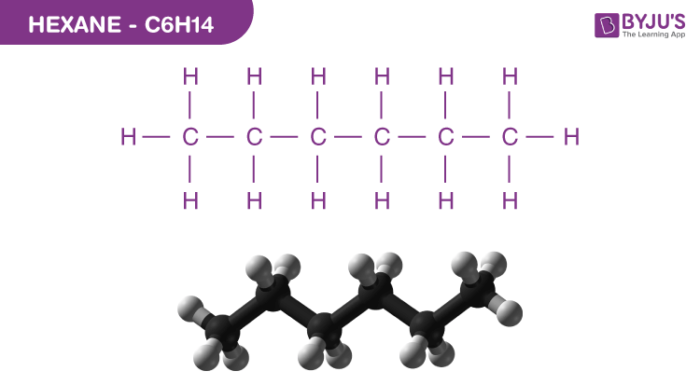
Physical Properties of Hexane – C6H14
| Odour | Gasoline-like odour |
| Appearance | Colourless volatile liquid |
| Complexity | 12 |
| Vapour Pressure | 17.60 kPa (at 20.0 °C) |
| Viscosity | 3.26 X 10-4 Pa-s at 20 deg C |
| Solubility | 9.5 mg L−1 |
Chemical Properties of Hexane – C6H14
-
- Hexane undergoes combustion reactions readily to form carbon dioxide and water molecules.
2C6H14 + 19O2 → 12CO2 + 14H2O
-
- Hexane being a higher hydrocarbon undergo thermal cracking forms more than one hydrocarbon.
C6H14 (on thermal cracking) → C4H10(butane) + C2H4(ethene)
Uses of Hexane – C6H14
- One of the most commonly used solvents in synthetic reactions using lipases.
- Used in biodiesel production.
- Hexane in commercial grades is used as solvents for varnishes, inks and adhesives.
- Used as a min solvent for vegetable oilseed and other non-petroleum oil extraction since the 1940s.
- Hexane azeotropes have been used for secondary extraction of residual lipids from hexane-extracted meals in order to improve flavour and odour.
Health Hazard
- Inhalation causes cough, respiratory track irritation, mild depression, cardiac arrhythmias.
- Ingestion causes vomiting, nausea, headache, swelling of abdomen and depression.
- Aspiration causes severe lung irritation, coughing, pulmonary edema, excitement followed by depression.
Frequently Asked Questions
What is hexane used for?
Hexanes are used for the manufacture of clothing, leather goods, and roofing glues. They are also used to extract cooking oils from seeds (such as canola oil or soy oil), to clean and degrease a variety of products, and in textile manufacturing.
Is hexane a good solvent?
When you are trying to dissolve a nonpolar compound, hexane is a strong solvent; but, if you were trying to use hexane to dissolve a polar compound it would be very unsuccessful. With a polar compound, water would be a safer option than hexane, because water is polar and can interact more readily with the polar compound.
Why hexane is flammable?
N-Hexane is a crude oil-producing compound. Pure n-Hexane is a liquid with a slightly unpleasant odour. It is extremely flammable, and can be explosive in its vapours. The key application for n-Hexane-containing solvents is to extract vegetable oils from crops such as soybeans.
Does hexane evaporate?
Pure n-hexane is a liquid with a slightly unpleasant odour. This evaporates very quickly into the sun, and just partially dissolves in water. N-Hexane is extremely flammable and can have volatile vapours.
Why is hexane used for extraction?
N-hexane is used as a solvent in solvent extraction for its attributes such as quick recovery, non-polar existence, low vaporisation latent heat (330 kJ / kg), and high solvent selectivity. Enzyme processing has significant potential to extract oil in the oilseed industry.

Comments