Definition
The ionic strength of a solution is the amount of ion concentration in it. It’s written in the form of I. It has an impact on ion activity. The ion interaction with water and other ions in the solution is marked. The ionic strength formula is used to calculate half of each ionic species’ total concentration.
The formula for ionic strength is as follows: 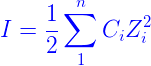
where, Ci – ionic concentration
Zi – ion charges
Table of Contents
- Ionic Strength: Definition and Unit
- The General Formula of Ionic Strength
- Ionic Strength Examples
- Importance and Uses of Ionic Strength
- Frequently Asked Questions
Ionic Strength: Definition and Unit
Different textbooks and monographs define the word ionic strength differently. Some use the SI unit mol kg-1 to determine it, whereas others use the non-SI unit mol L-1. Computing means ionic activity coefficients are complicated by the conversion to the SI unit mol m3.
There’s also a dimensionless ionic strength unit. Students find the idea of ionic strength particularly perplexing due to the multiple definitions, especially when utilising it to determine mean activity coefficients using the Debye-Hückel theory. To clear up this ambiguity, it is suggested that ionic strength be defined as a dimensionless quantity.
The General Formula of Ionic Strength
The formula for calculating ionic strength is the sum of each ion’s molar concentration multiplied by the valence squared.
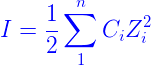
where 1/2 is because both ions (cation and anion) are taken into account, C is the concentration in molar units (mol/L), and Z is the charge of each ion. For example, if the ions are sulphate (SO42-), for example, Z = 2. As can be seen, the multivalent ion makes a more significant contribution.
Ionic Strength Examples
Example 1: Calculate the ionic strength of a 3M potassium chloride solution.
To begin, the dissociation must be drawn: KCl → K+ + Cl–
As a result, each ion has the same concentration as the salt, 3 mol/L.
The equation can then be used:
I = 1/2 [(3 mol/L)(+1)2 + (3 mol/L)(-1)2] = 3M
Hence, the ionic strength is 3M.
Example 2: Calculate the ionic strength of a potassium chloride solution of 1M and magnesium sulphate of 0.2M.
First, the dissociation must be drawn: KCl → K+ + Cl– and MgSO4 → Mg2+ + SO42-.
Now applying the equation,
I = 1/2 [(1 mol/L)(+1)2 + (1 mol/L)(-1)2 + (0.2 mol/L)(+2)2 + (0.2 mol/L)(-2)2 ] = 3.6 M
Thus, the ionic strength is 3.6 M.
Ionic Strength Considerations
Non-ideal solutions are those that do not conform to the ideal behaviour. Solutions that need consideration of interaction forces are a good example. Above all, the unit of molality (mol/kg of H2O) is used here rather than molarity.
Importance and Uses of Ionic Strength
The Debye–Hückel theory, which describes the severe departures from ideality common in ionic solutions, relies heavily on ionic strength. It’s also crucial for the theory of double layers in colloids and other heterogeneous systems and related electrokinetic and electroacoustic phenomena.
To reduce changes in the activity quotient of solutes at lower concentrations throughout a titration, high-ionic-strength media are utilised in stability constant determination. Due to the presence of dissolved salts, natural waters such as mineral water and seawater typically have a non-negligible ionic strength, which has a substantial impact on their properties.
In theoretical chemistry, ionic strength is used to calculate salt dissociation in heterogeneous systems like colloids. It’s also used in biochemistry and molecular biology to determine the strength of buffer solutions with concentrations that should be close to those found in nature.
Frequently Asked Questions on Ionic Strength Unit
How do you measure ionic strength?
Ionic strength is typically calculated as the product of a given ion’s concentration, ci, and its charge, Zi, summed over all ions in solution, divided by two, and measured either as mass per unit volume (i.e., mg/L) or in moles (i.e., mmol/L) (IUPAC Quantities, Units and Symbols in Physical Chemistry, 1993).
What is the importance of ionic strength?
Chemists require an understanding of ionic strength. This is due to the ions’ electrical charge, which can attract or repel them. Furthermore, this attraction and repulsion cause ions to behave in specific ways.
State the unit of strength in chemistry.
The mass of solute (in grammes) dissolved in one litre of solution is the strength of the solution. The strength units are gL–1 or g dm–3.
What is the effect of ionic strength on solubility?
Ionic strength can affect protein behaviour, causing salting in (increasing solubility) or salting-out (increased solubility) (decreased solubility). The colloidal stability is frequently blamed for a decrease in solubility as ionic strength rises.
What does it imply to have a low ionic strength?
Low ionic strength generally reduces zeta potential, but not to the point where the hydrophobic effect kicks in. Before this, any rise in ionic strength boosts the number of protein molecules that the solvent can hold.

Comments