What is Lead iodide?
PbI2 is a salt with the chemical name Lead iodide. It is also called Plumbous iodide or Lead (II) iodide or Lead diiodide. It is a good oxidizing agent and burns with a green-coloured flame. It is widely used in printing, photography, and seed clouds.
Lead diiodide is a crystalline solid, odourless and yellow in colour. It is insoluble in water and denser when compared to water. It is a threat to the environment and immediate steps need to be taken to stop the spread.
Properties of Lead iodide – PbI2
| PbI2 | Lead iodide |
| Molecular weight of PbI2 | 461.01 g/mol |
| Density of Lead iodide | 6.16 g/dm3 |
| Melting point of Lead iodide | 402 °C |
| Boiling point of Lead iodide | 953 °C |
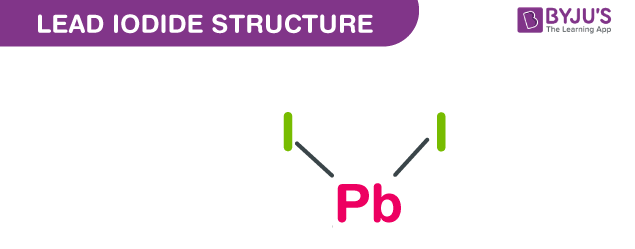
Lead iodide structure – PbI2
- Lead iodide is used as a precursor in the fabrication of solar cells.
- Used as an organic solvent.
- Used as photon detector for X-rays and gamma-rays.
- Used in the manufacturing of thermoelectric materials.
- Used in photography.
- Used in printing.
- Used in bronzing.
- Used in making mercury vapour arc lamps.
- Used to record optical images in films.
Production of Lead iodide
Plumbous iodide is commonly produced by a double displacement reaction between lead nitrate and potassium iodide in water.
Pb(NO3)2 + 2 KI → PbI2 + 2 KNO3
At room temperature, potassium nitrate is soluble, whereas lead iodide is insoluble, and therefore it undergoes precipitation.
It can also be produced by reacting iodine vapour with molten lead at a temperature range of 500 to 700 °C.
Another method to obtain lead iodide in the form of a thin film is by depositing a film of PbS and exposing it to iodine vapour. The reaction is as follows:
PbS + I2 → PbI2 + S
The sulfur (S) obtained is washed with dimethyl sulfoxide (C2H6OS).
Health hazards
Some of the early symptoms that occur due to swallowing or inhaling Lead (II) iodide are gastrointestinal disorders, constipation, weakness, paralysis. Ingesting large amounts of this compound results in irritation of the alimentary tract. It is non-combustible and when heated to decompose, it produces toxic and corrosive fumes.
Learn more about the Structure, and physical and chemical properties of PbI2 along with the reaction between iodide ions and hydrogen peroxide from the experts at BYJU’S.

Comments