Lucas test is used to differentiate and categorize primary, secondary and tertiary alcohols using a solution of anhydrous zinc chloride in concentrated hydrochloric acid. This solution is commonly referred to as the Lucas reagent.
What is the Lucas Test?
Primary, Secondary and Tertiary Alcohols are classified based on their reactivity with the Lucas reagent. The reaction that occurs in the Lucas test can be seen as a nucleophilic substitution reaction. In this reaction, the Chloride in the zinc-chloride bond is replaced with a hydroxyl group originating from the given alcohol.
The reaction displays the difference in reactivity of the different types of alcohol as well as the difference in the ease at which corresponding carbocations of the alcohols are formed. For example, primary alcohols do not react readily at room temperature with the added Lucas reagent whereas tertiary alcohols react immediately.
The observation of a change where the clear and colourless characteristic of the solution changes to a turbid, cloudy, and hazy one implies that a chloroalkane has formed. This observation is a positive indication for the Lucas test.
Primary, Secondary, and Tertiary alcohols react with the Lucas reagent to form the chloroalkane at different rates. Tertiary alcohols react the fastest due to the fact that organic chloride has relatively low solubility in the aqueous mixture.
Lucas Test for Primary, Secondary, and Tertiary Alcohols
As discussed earlier, the test can be used to differentiate the reaction speed of the alcohol with the given Lucas reagent. This is done by measuring the time taken for the clear solution to turn turbid.
Given below is a table describing the positive Lucas test observations for different types of alcohols.
| Primary Alcohol | The solution remains colourless unless it is subjected to heat. The solution forms an oily layer when heated. Example: 1-Pentanol. |
| Secondary Alcohol | The solution turns turbid and forms an oily layer in three to five minutes (varies based on the solubility). Example: 2-Pentanol. |
| Tertiary Alcohol | The solution turns turbid and forms an oily layer immediately. Example: 2-methyl-2-butanol. |
Lucas Test Mechanism
The mechanism followed in this reaction is an SN1 nucleophilic substitution. It can be broken down into the following two steps.
Step 1
The OH group belonging to the alcohol is protonated by hydrochloric acid. Now, since chlorine is a stronger nucleophile than water, it replaces the resulting water molecule attached to the carbon. This leads to the formation of a carbocation.
Step 2
The chloride anion now attacks the carbocation and forms an alkyl chloride. This alkyl chloride is insoluble and hence turns the solution turbid. The net mechanism of the Lucas test can be illustrated as follows.
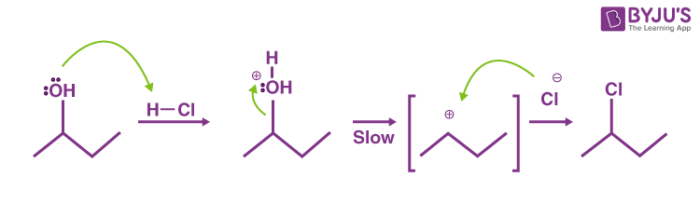
Thus, the primary, secondary, and tertiary alcohols can be differentiated based on the rate at which they turn the solution turbid when reacted with the Lucas reagent.

Comments