What is Oppenauer Oxidation?
Oppenauer Oxidation is the process of conversion of secondary alcohols to ketones by selective oxidation. This reaction is named after Rupert Viktor Oppenauer. Oxidation reaction takes place in the presence of [Al(i-Pro)3] in excess of acetone.
It is an aluminium alkoxide catalysed oxidation of a secondary alcohol to the corresponding ketone. This is the reverse of the Meerwein Ponndorf Verley reduction. It is a very good method to oxidise allylic alcohols to α, β- unsaturated ketones.
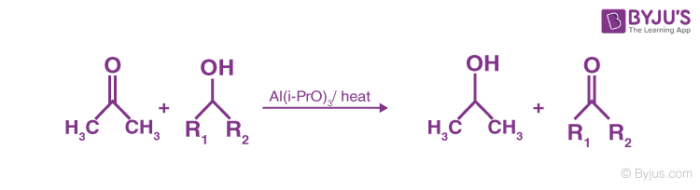
Table of Contents
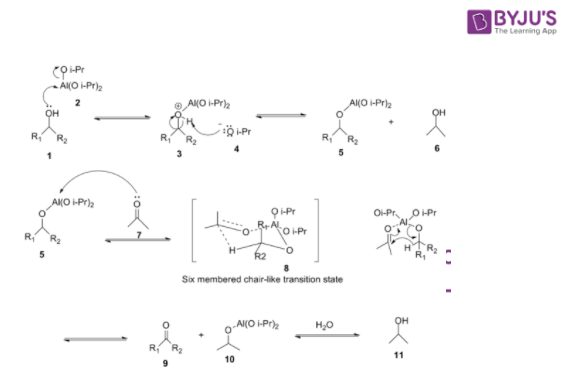
Oppenauer Oxidation Mechanism
- In the first step, alcohol coordinates with aluminium isopropoxide to form a complex
- This complex reacts with a ketone to form a six-membered transition complex
- The alpha-carbon of the alcohol is converted to the carbonyl carbon from the aluminium-catalysed hydride shift.
- The acetone proceeds over a six-membered transition state.
- The desired ketone is formed after the hydride transfer
This reaction is used to oxidise alcohols to carbonyl compounds. For example, simple ketone acetone or cyclohexanones is used as the hydride acceptor in the presence of aluminium alkoxide, usually isopropoxide or t-butoxide. The oxidation is the exact reverse of Meerwein-Ponndorf-Verley reduction and involves deprotonation of the alcohol by equilibration with the alkoxide followed by hydride transfer. This equilibrium is generally displaced to the right by using a large excess of the hydride acceptor.
Disadvantages of Oppenauer oxidation
The classical Oppenauer oxidation is a highly chemoselective oxidation process but the disadvantages of this method include the use of high temperatures, large quantities of ketone hydride acceptors and the production of aldol condensation products with the hydride acceptors. Research has focused on the development of more efficient Oppenauer type oxidations that may be performed under milder conditions.
For example, aluminium compounds involved in the oxidation are basic and consequently prototropic shifts can take place within the product. Thus, during the oxidation of cholesterol, the C=C migrates to give α, β – unsaturated ketones.
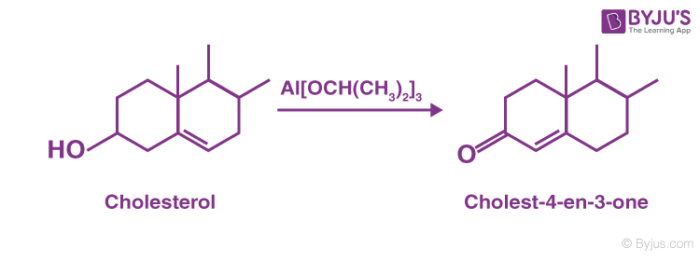
Oppenauer Oxidation is not a good method to prepare aldehydes since the basic medium induces reactions between aldehydes and ketones eg. aldol condensation. Oppenauer type oxidations may be performed under milder conditions.
Frequently Asked Questions on Oppenauer Oxidation
What is meant by oxidation reaction?
Oxidation is any chemical reaction involving the passage of electrons. This forms a chemical called rust when iron reacts with oxygen because it has been oxidised (the iron has lost some electrons) and the oxygen has been reduced (the oxygen has gained some electrons).
What causes oxidation?
Oxygen and atmospheric moisture are the major players in corrosion and oxidation. It is a metal surface’s chemical reaction with oxygen that causes some metal to corrode and form the surface’s oxidation or better known as metal oxide.
What is secondary alcohol?
Secondary alcohol is a compound in which a hydroxy moleucle, —OH, is bound to a saturated carbon atom with two other carbon atoms.
Can secondary alcohol be oxidised?
Secondary alcohols are ketones oxidised-and that’s it. For example, if you heat the secondary alcohol propan-2-ol with a diluted sulphuric acid solution of sodium or potassium dichromate(VI), you can form propanone.
What is the functional group of a ketone?
Aldehydes and ketones are organic compounds that include a functional group of carbonyls, C = O. This group’s carbon atom has two remaining bonds that may be occupied by substitutes for hydrogen, alkyl, or aryl. If hydrogen is at least one of these replacements, the compound is an aldehyde.
What happens when primary alcohol is oxidised?
Based on reaction conditions, primary alcohols may be oxidised into either aldehydes or carboxylic acids. As carboxylic acids are formed, the alcohol is first oxidised into an aldehyde and then further oxidised into the acid.
Why is the oxidation of alcohol important?
A significant step in the degradation of fats during human metabolism (e.g. L-malate in oxaloacetate) is the oxidation of alcohol groups into carbonyl groups. It is also possible to provide a reverse reaction in which NADH serves as a biological hydride donor and reducing agent.
