What is Partition Chromatography?
Partition Chromatography technique is defined as
the separation of components between two liquid phases viz original solvent and the film of solvent used in the column.
This separation theory was introduced in the year 1940s which was published by Richard Laurence Millington Synge and Archer Martin. It is also known as Liquid-liquid chromatography (LLC). Or if gas is the mobile phase it is called Gas-liquid chromatography (GLC).
Table of Contents
Principle of Chromatography
-
-
-
- Chromatography is a separation method where the analyte is contained within a liquid or gaseous mobile phase, which is pumped through a stationary phase.
- Usually, one phase is hydrophilic and the other lipophilic. The components of the analyte interact differently with these two phases.
- Depending on the polarity they spend more or less time interacting with the stationary phase and are thus retarded to a greater or lesser extent.
- This leads to the separation of the different components present in the sample.
-
-
Partition Chromatography Principle
The separation of the components from the sample mixture is carried out by the process of partition of the components between 2 phases. Both phases are in liquid form. In this process, the immiscible solid surface coated with the liquid surface on the stationary phase is in the mobile phase. The liquid surface is immobilised by a stationary phase which results in making it a stationary phase. The mobile phase moves from the stationary phase and components get separated. The separation depends on different partition coefficients.
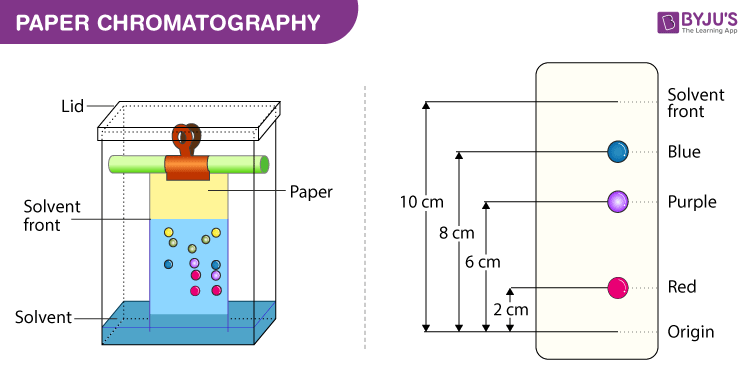
Partition Chromatography Diagram
Partition Chromatography Procedure
Below we have explained the procedure to conduct Paper Chromatography Experiment for easy understanding
Apparatus required – chromatography jar, liquid impregnated paper (stationary phase), capillary tube (to apply sample mixture), mobile phase (example chloroform, methanol, acetone, ethanol).
-
-
-
- Take a clean and dry chromatography jar.
- To make sure that the environment of the jar is saturated with solvent vapours, a paper impregnated in the mobile phase is set to the walls.
- Add the mobile phase to the jar. Around 0.5 cm to 1 cm from the bottom of the jar.
- Close the jar.
- Allow attaining equilibrium.
- Mark the baseline on the adsorbent.
- Apply sample to the paper with the help of a capillary tube.
- Air-dry the sample spot.
- Place the paper in the jar and close it.
- Allow the system to stand till the solvent moves to some distance from the baseline.
- Take out the paper and dry it.
- If the sample components are separated, showing colours, then dry them in ordinary light. If it is a colourless component, then dry it in a UV lamp.
- Store the chromatogram
- Calculate the Rf value.
-
-
Partition Chromatography Applications
There are various applications of Paper Chromatography. Some of the applications are mentioned below:
-
-
-
- To separate and identify amino acids.
- To separate and identify tannins.
- To separate and identify alkaloids.
- To separate and identify carbohydrates.
- To separate and identify glycosides.
-
-
Types of Partition Chromatography
-
-
-
- Liquid-liquid Chromatography – It is a chromatography technique where a sheet of blotting paper, is used instead of adsorption column. The components are separated based on their differential migratory velocities. On separating, they are stained to make the chromatogram visible.
- Gas-liquid Chromatography – A chromatography technique in which the separation of the mixture is done by an inert gas along a tube. The tube is filled with finely divided inert solid. The solid is coated with a nonvolatile oil. The migration of each component occurs at a rate determined by its solubility in oil as well as its vapour pressure.
-
-
Application of partition chromatography
-
-
-
- Remove the detergent from the protein solution.
- Steroids, bile acids, and mycotoxins are separated.
- Pesticides, phenols, and insecticides are all being removed.
- Concentration of trace metals in aqueous solutions
-
-
Frequently Asked Questions
Where is chromatography used?
Chromatography is used in industrial processes to purify chemicals, test trace quantities of substances, separate chiral compounds and quality control test products. Chromatography is the physical process of separating or analyzing complex mixtures.
What is the Rf value?
The Rf value is defined as the ratio of the distance moved by the solvent (i.e. the colouring or pigment being tested) and the distance moved by the solvent (known as the Solvent front) along the paper, where both distances are measured from the common origin or application baseline.
Who is the father of chromatography?
The Italian-born Russian botanist, Mikhail Semyonovich Tswett (1872-1919), is considered to be the Father of Chromatography.
What is the main purpose of chromatography?
Preparative chromatography is intended to isolate the components of a mixture for subsequent use and is, therefore, a method of purification. Analytical chromatography is usually done with smaller amounts of material and is intended to determine the presence of the relative proportions of analytes in a mixture.
What are the advantages of chromatography?
Using chromatography, precise separation, analysis, and purification are possible. It works on a variety of samples including drugs, food particles, plastics, pesticides, samples of air and water, and extracts of tissue.
To learn more about the different types of Partition Chromatography from the experts, register to BYJU’S now!
Other important links:
| Column chromatography | Thin layer chromatography |

This app is very helpful