This article provides a brief introduction to the preparation of phenols from haloarenes, benzene sulphonic acid, diazonium salts, and cumene. Phenol is an organic compound containing a benzene ring bonded to a hydroxyl group. Phenols are weak acids and generally form phenoxide ions by losing one positive hydrogen ion (H+) from the hydroxyl group.
Earlier, phenol was primarily synthesized from coal tar. However, with advancements in technology, several new methods have been devised for the preparation of phenols. In laboratories, phenol is primarily synthesized from benzene derivatives. Some of the methods of preparation of phenols are explained below.
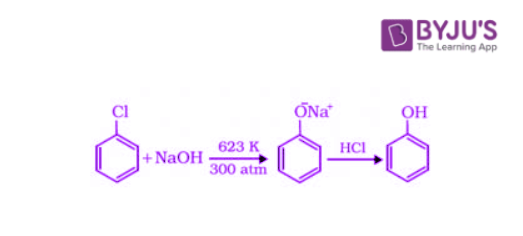
1. Preparation of Phenols from Haloarenes
Chlorobenzene is an example of a haloarene which is formed by the monosubstitution of the benzene ring. When chlorobenzene is fused with sodium hydroxide at 623K and 320 atm, sodium phenoxide is produced. Finally, sodium phenoxide on acidification gives phenol.
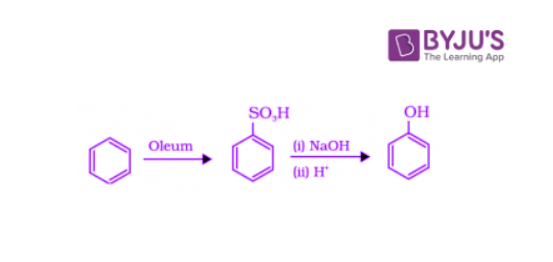
2. Preparation of Phenols from Benzene Sulphonic Acid
Benzenesulphonic acid can be obtained from benzene by reacting it with oleum. This benzene sulphonic acid can be treated with molten sodium hydroxide at high temperatures to encourage the formation of sodium phenoxide. Finally, sodium phenoxide on acidification gives phenol.
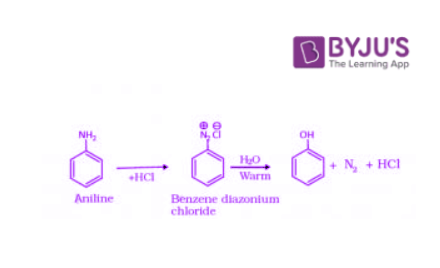
3. Preparation of Phenols from Diazonium Salts
When an aromatic primary amine is treated with nitrous (NaNO2 + HCl) acid at 273 – 278 K, diazonium salts are obtained. These diazonium salts are highly reactive in nature. Upon warming with water, these diazonium salts finally hydrolyze to phenols. Phenols can also be obtained from diazonium salts by treating it with dilute acids.
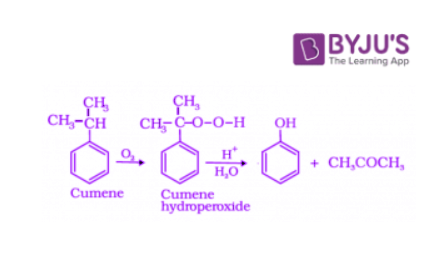
4. Preparation of Phenols from Cumene
Cumene is an organic compound obtained by Friedel-Crafts alkylation of benzene with propylene. Upon oxidation of cumene (isopropylbenzene) in the presence of air, cumene hydroperoxide is obtained. Upon further treatment of cumene hydroperoxide with dilute acid, phenols are obtained. Acetone is also produced as one of the by-products of this reaction in large quantities. Hence, phenols prepared by these methods need purifications.
For detailed discussions on the preparation of phenols, please download the BYJUS app.

Comments