What is Potassium Bromide?
KBr is a salt which is widely used as a sedative and as an anticonvulsant with the chemical name Potassium Bromide.
Potassium Bromide is also called Bromide salt of potassium or Kalii bromidum or Tripotassium tribromide.
The potassium bromide salt is odourless and comes in the form of white crystalline powder, colourless crystals, or white granular solid with a pungent bitter saline taste. Aqueous solutions of KBr have a pH value of 7.
Table of Contents
- Properties of Potassium Bromide – KBr
- Potassium Bromide Structure – KBr
- KBr Uses (Potassium Bromide)
- Production of Potassium Bromide
- Potassium Bromide Reactions
- Health Hazards
- Frequently Asked Questions
Properties of Potassium Bromide – KBr
| KBr | Potassium Bromide |
| Molecular weight of KBr | 119.002 g/mol |
| Density of Potassium Bromide | 2.74 g/cm3 |
| Melting point of Potassium Bromide | 734 °C |
| Boiling point of Potassium Bromide | 1,435 °C |
Potassium Bromide Structure – KBr
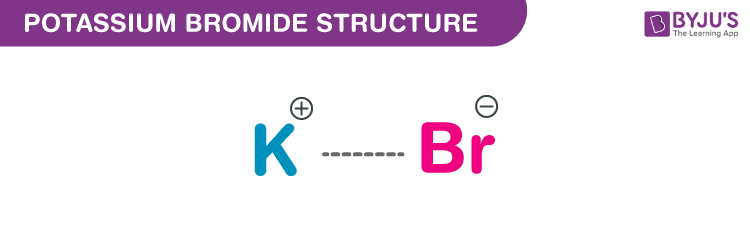
Potassium Bromide Structure – KBr
KBr Uses (Potassium Bromide)
- Potassium Bromide is used to manufacture photographic papers and plates.
- Used as a laboratory agent.
- Used as a heat stabiliser for nylon.
- Used as a Sedative.
- Used as an anticonvulsant.
- Used in the water treatment of aquariums
- Used to manufacture chemicals.
- Used as plasticizers.
Production of Potassium Bromide
One of the traditional methods of producing KBr is by reacting potassium carbonate with an iron (III, II) bromide, Fe3Br8, produced by treating scrap iron in water with excess bromine. The chemical equation for the same is given as follows:
4 K2CO3 + Fe3Br8 → 8 KBr + Fe3O4 + 4 CO2
Potassium Bromide Reactions
At a near pH value of seven, potassium bromide gets fully dissociated thus, it is an ionic solid. This reaction plays an important role in the manufacture of silver bromide for photographic films. The chemical equation for the same is shown as:
KBr(aq) + AgNO3(aq) → AgBr(s) + KNO3(aq)
The reaction of bromide in its aqueous form with metal halides produces complex.
For example, the reaction of potassium bromide and copper(II) bromide produces a complex compound. The chemical equation for the same is given as;
2 KBr(aq) + CuBr2(aq) → K2[CuBr4](aq)
Health Hazards
1. Some of the symptoms include vomiting, ataxia, coma, irritability, and mental confusion.
2. It can cause mania, skin rashes, drowsiness, and hallucinations.
3. It also causes neurological signs, increased spinal fluid pressures, death, vertigo, and sensory disturbances.
Frequently Asked Questions
What is potassium bromide used for?
Potassium bromide is one of the standard anticonvulsant drugs used to treat canine and feline epilepsy and is often abbreviated as KBr. This is often used in combination with Phenobarbital but can also be used by itself to regulate seizure activity.
How does potassium bromide work?
Potassium bromide acts as chloride ions to fight for entry to brain tissues. If the bromide levels in the brain increase and the chloride levels fall, electrical activity in the central nervous system is disrupted, making it difficult to cause a seizure.
How is potassium bromide used?
What is the colour of potassium bromide solution?
Potassium bromide is a pure, white crystalline powder under normal conditions.
What occurs when potassium bromide KBr is dissolved in water?
If KBr, potassium bromide, is dissolved in water it dissociates into ions of potassium, K+ and bromine, Br–ions. The water molecules surround these ions to create a surface layer.
Learn more about the Structure, physical and chemical properties of KBr from the experts at BYJU’S.

Comments