The particles that make up matter are very small. To the naked eye, these particles are undetectable. Matter has its own set of properties. Physical features include colour, odour, melting point, boiling point, density, and so on, while chemical properties include composition, combustibility, reactivity with acids and bases, and so on. In our environment, for example, a book, a pen, all living things, including pencils, water, and air, are made up of matter.
|
Definition: Matter is defined as anything that occupies space and has volume.Solid, liquid, and gas are the three physical states in which matter can exist. |
Properties of Matter Chemistry Questions with Solutions
Q-1: Which of the following is not a fluid form of matter?
- Solids
- Liquids
- Gases
- None of the above
Answer: a) Solids
Explanation:
Liquids and Gases are the fluid form of matter, because they can flow or move.
Solids are the rigid form of matter; there is not much freedom of movement.
Q-2: A few substances are grouped in increasing order of their particle’s ‘forces of attraction.’ Which of the following is the correct order?
- Water,oxygen,chalk
- Salt,juice,wind
- Nitrogen,water, sugar
- Air, salt, oil
Answer: c) Nitrogen,water, sugar
Explanation:
Particles in solids are close together in an ordered manner, with little room for mobility. Particles in liquids are close together but have the ability to move about. In contrast to solid or liquid phases, gases have far apart particles that move easily and quickly.
This means that solids have strong forces of attraction between particles, whereas gases have weak forces of attraction. Liquids, on the other hand, are halfway between solids and liquids.
Solids: Chalk,salt and sugar
Liquids: Water, juice and oil
Gases: Nitrogen,oxygen,wind and air.
The correct order will be Nitrogen<Water<Sugar
Q-3: A form of matter has no fixed shape and no fixed volume. An example of this form of matter is:
- Petrol
- Iron
- Krypton
- Carbon steel
Answer: c) Krypton
Explanation: Gases have neither a fixed volume nor a fixed shape. They take up the entire volume of the container in which they are placed. Krypton is a kind of gas. Petrol is a liquid. Solids are iron and steel.
Q-4: Explain why we can simply move our hand in the air but need a karate expert to accomplish the same with a piece of wood.
Answer: In air, there will be a weak attraction between the particles. As a result, we can move our hands in the air, whereas the particles in a solid plank are tightly packed and have a strong attraction between them. To overcome the pull, a karate practitioner must use a tremendous amount of force.
Q-5: A tea bag immersed in a cup of hot water changes its colour. Name the property responsible for it.
Answer: Diffusion.
Diffusion is the mingling of two substances caused by the movement of their particles. A tea bag submerged in hot water will diffuse and affect the colour of the water.
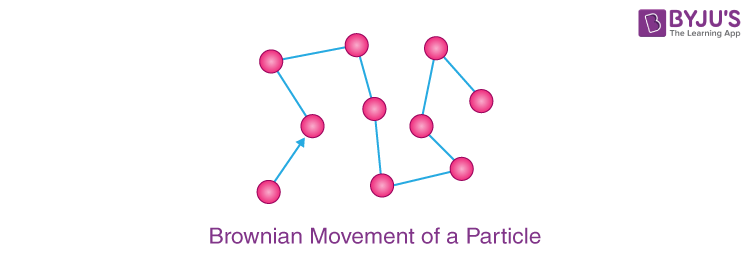
Q-6: State the characteristic of matter demonstrated by the Brownian movement? Draw a diagram to show the movement of a particle during Brownian movement?
Answer: Brownian motion, also known as Brownian movement, is the zig-zag movement of tiny particles suspended in fluid form of matter.
It demonstrates the following properties of matter:
i) Matter particles are extremely tiny.
(ii) Matter particles are in continual motion.
Q-7: Fill in the blanks with the suitable words.
(a) ____________ properties can be measured or observed without changing the identity or the composition of the substance.
(b) Diffusion of solids are ____________ than liquids.
(c) ___________ drug is used for helping AIDS patients.
(d) A gas on cooling liquifies to the____________.
(e) The three states of matter are interconvertible by changing the conditions of _______ and _______.
Answers:
- Physical
- Lesser
- Azidothymidine
- Liquid
- Temperature, pressure
Q-8: “ Materials contain at least two pure substances and show the properties of the constituents”. Identify whether this statement applies to an element, a compound, or a mixture.
Answer: Mixtures are physical combinations of two or more pure substances where the properties of the constituents are retained. They can be separated by physical methods of separation.
Q-9: Pounding a coal into little particles is possible, but hammering an aluminium piece into small particles is impossible. Which property of matter particles is demonstrated by these observations?
Answer: Matter is made up of small partic;es which attract each other. The attraction varies from material to material. When hammered, coal breaks easily, but aluminium does not, indicating that the particles of aluminium attract each other with more power.
Q-10: The scientist who used a microscope to study the movement of pollen grains suspended in water is
- J.J Thomson
- Robert Brown
- John Dalton
- Ernest Rutherford
Answer: b) Robert Brown
Explanation: Robert Brown studies the movement of pollen grains suspended in water using a microscope. Pollen grains were discovered to be travelling quickly and irregularly across water. Brownian motion describes this occurrence.
Q-11: Match column I with column II.
|
Column I |
Column II |
|---|---|
|
Gases |
Particles are in continual motion |
|
Matter |
Neon |
|
A Refrigerant |
Compressible |
|
An Element |
Washing soda |
|
A compound |
Freon |
Answer:
|
Column I |
Column II |
|---|---|
|
Gases |
Compressible |
|
Matter |
Particles are in continual motion |
|
A Refrigerant |
Freon |
|
An Element |
Neon |
|
A compound |
Washing soda |
Q-12: Which of the following diagrams is accurate for 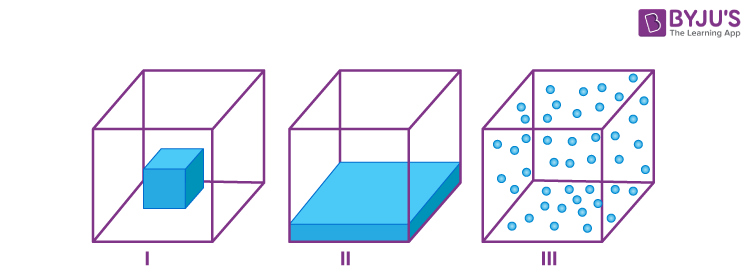
- A liquid
- A solid
- A gas
Give the proper justifications for your response.
Answer:
- II
- I
- III
Explanation:
A solid is a rigid body with a fixed shape and volume.
A liquid has fixed volume but not shape. It takes on the shape of the container it’s poured into.
A gas has no definite shape and volume.
Q-13: Place a solid object in a graduated cylinder with water and measure how much water is displaced to determine its,
- Mass
- Density
- Volume
- Weight
Answer: c) Volume
Explanation: A graduated cylinder is filled with enough water to cover the object, and the volume is measured. The object is placed in the cylinder, and the volume is measured once more. The object’s volume is the difference between the two volumes.
Q-14: Combustibility is a
- Physical property
- Reactive Property
- Chemical Property
- Not a property
Answer: c) Chemical property
Explanation: A chemical change is required for the measurement or observation of chemical properties. Physical qualities can be measured without a chemical change occurring. Chemical characteristics include acidity or basicity, combustibility etc.
Q-15: Which is correct when given a coffee and water solution, as well as water and pepper.
a. Pepper has the same solubility as coffee when stirred.
b. Pepper is water soluble, while coffee is not.
c. While coffee dissolves in water, pepper does not.
d. The solubility of coffee and pepper is the same.
Answer: c) While coffee dissolves in water, pepper does not.
Explanation: Salt, sugar, and coffee all dissolve in water. In hot water, they usually dissolve faster and better. Pepper and sand are insoluble, meaning they won’t dissolve even in boiling water.
Practise Questions on Properties Of Matter
Q-1: At 1000C , what happens to the water
- It condenses
- It freezes
- It boils
- It melts
Q-2: Which is an example of a reversible form of matter?
- Egg
- Fire
- Ice
- Wood
Q-3: Which of the following cannot be classified as a matter?
- Heat
- Air
- Paper
- Wood
Q-4: What do you mean by intermolecular spaces? How does it vary in different states of matter?
Q-5: Substance P has a definite volume but no particular shape and is fluid at -80 degrees Celsius. Substance P has no definite volume or shape at -55 degrees Celsius. Which of the following could be substance P’s melting and boiling points?
|
S.No |
Melting point(0C) |
Boiling Point(0C |
|---|---|---|
|
a) |
-92 |
-60 |
|
b) |
-85 |
-40 |
|
c) |
-80 |
-56 |
|
d) |
-70 |
-50 |
Click the PDF to check the answers for Practice Questions.
Download PDF
Recommended Videos
What Is Matter?

Characteristics of Particles of Matter
Comments