Table of Contents
What is Sodium Dichromate?
Na2Cr2O7 is an inorganic compound with the chemical name Sodium dichromate. It is also called Bichromate of soda or Disodium dichromate or Sodium dichromate (VI). It is a powerful oxidizing agent and highly corrosive.
Bichromate of soda is a red to red-orange crystalline solid. It is odourless and dissolves in water, methanol as well as ethanol. It is widely used as a corrosion inhibitor. On heating, it liberates toxic chromium fumes.
Properties of Sodium Dichromate – Na2Cr2O7
| Na2Cr2O7 | Sodium dichromate |
| Molecular weight of Na2Cr2O7 | 261.97 g/mol (anhydrous) |
| Density of Sodium dichromate | 2.52 g/dm3 |
| Melting point of Sodium dichromate | 356.7 °C |
| Boiling point of Sodium dichromate | 400 °C |
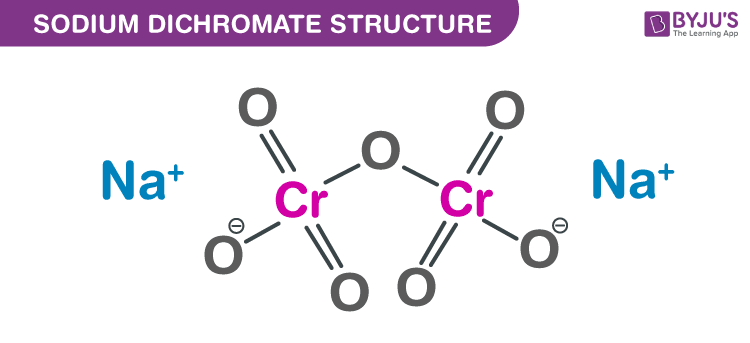
Sodium Dichromate structure – Na2Cr2O7
Na2Cr2O7 Uses (Sodium Dichromate)
-
-
-
- Sodium dichromate is a primary material in the manufacturing of chromium chemicals.
- Used as textile dyes.
- Used in water treatment.
- Used in leather tanning.
- Used as a catalyst.
- Used as an oxidising agent in the manufacturing of synthetic organic chemicals.
- Used in the refining of petroleum.
- Used in metal treatments such as electro-engraving of copper.
- Used in Colorimetry experiment to determine the amount of copper.
- Used as an insecticide and fungicide.
- Used as a colourant in the glass.
-
-
Production of Sodium Dichromate
On a large scale, Disodium dichromate is obtained from ores that contain chromium(III) oxides.
Step 1: Fusing the ore with a base such as sodium carbonate
Step 2: Maintain a temperature at around 1000 °C
Step 3: Conduct the above two steps in the presence of air which is the source of oxygen. The reaction is as follows:
2Cr2O3 + 4Na2CO3 + 3O2 → 4Na2CrO4 + 4CO2
The above steps solubilize the chromium and allow its extraction in the hot water. The other components present in the ore are poorly soluble hence acidification of the resulting aqueous extract with the help of carbon dioxide or sulfuric acid produces dichromate.
2Na2CrO4 + 4CO2 + H2O → Na2CrO7 + 2NaHCO3
2Na2CrO4 + H2SO4 → Na2CrO7 + Na2SO4 + H2O
With the process of crystallization the dichromate is extracted as dihydrate.
Health hazards
Inhalation of dust or mist of Sodium dichromate (VI) causes respiratory irritation which resembles asthma and nasal septal perforation may occur. Swallowing causes diarrhoea, and vomiting. When exposed to eyes and skin it results in local irritation and dermatitis.
It is a non-combustible compound but increases the combustion of other compounds. It can explode and catch fire when comes in contact with combustible substances.
Frequently Asked Questions – FAQs
What is the chemical formula of Sodium dichromate?
What is the molecular weight of Sodium dichromate(Na2Cr2O7)?
What is the oxidation state of Chromium in Sodium dichromate?
Learn more about the Structure, physical and chemical properties of Na2Cr2O7 from the experts at BYJU’S.

Comments