Electrode potential is a measure of reducing power of any element. It is also called standard reduction potential. Standard Hydrogen Electrode is used as a reference electrode when calculating the standard electrode potential of a half cell.
Table of Contents
- What is a Standard Hydrogen Electrode?
- Recommended Videos
- Uses
- Standard Hydrogen Electrode Construction
- Standard Hydrogen Electrode Diagrams
- Solved Example
- Frequently Asked Questions – FAQs
What is a Standard Hydrogen Electrode?
The Standard Hydrogen Electrode is often abbreviated to SHE, and its standard electrode potential is declared to be 0 at a temperature of 298K. This is because it acts as a reference for comparison with any other electrode.
The half cell reaction of SHE can be written as follows:
2H+ (aq) + 2e– → H2 (g)
The reaction given above generally takes place on a platinum electrode. The pressure of the hydrogen gas present in this half cell equals 1 bar.
Recommended Videos

Uses of Platinum in the Standard Hydrogen Electrode
Platinum is used in the Standard Hydrogen Electrode due to the following reasons:
- Platinum is a relatively inert metal which does not corrode easily.
- Platinum has catalytic qualities which promote the proton reduction reaction.
- The surface of platinum can be covered with platinum black, a fine powder of platinum. This type of platinum electrode is called a platinized platinum electrode.
- Platinum also improves the reaction kinetics by adsorbing hydrogen at the interface.
Standard Hydrogen Electrode Construction
The parts that make up a Standard Hydrogen Electrode are listed below.
- A platinum electrode is covered in finely powdered platinum black (platinized platinum electrode).
- A hydrogen blow.
- A solution of acid having an H+ molarity of 1 mole per cubic decimeter.
- SHE also contains a hydroseal which is used to prevent the interference of oxygen.
- The other half-cell of the entire Galvanic cell must be attached to the Standard Hydrogen Electrode through a reservoir in order to create an ionically conductive path. This can be done through a direct connection, through a narrow tube, or even through the use of a salt bridge.
Standard Hydrogen Electrode Diagram
A labelled diagram of a standard hydrogen electrode is provided below. In SHE, a salt bridge is used to link SHE with the other half cell.
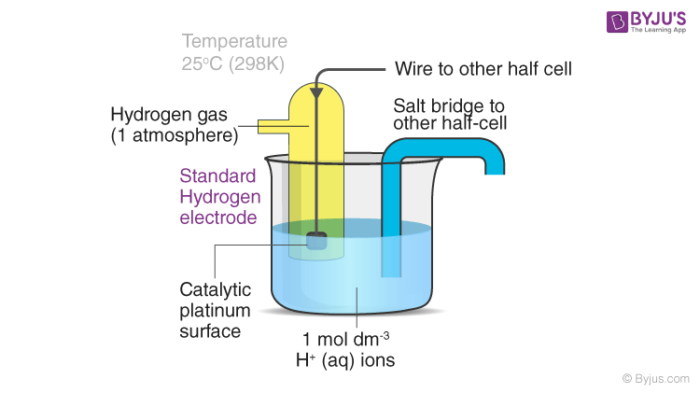
The platinized platinum surface has a very high adsorption activity. Therefore, this surface must be protected from atmospheric oxygen as well as from organic substances. Substances such as arsenic and sulphur compounds can deactivate or poison the catalyst.
Solved Example
Question:
The pressure of H2 required to make the potential of H2-electrode zero in pure water at 298 K is
Solution:
Frequently Asked Questions – FAQs
What is the use of a standard hydrogen electrode?
SHE is the basic guide for the reporting of the capacity of quantitative half-cells. It is a type of gas electrode and has been commonly used as a reference electrode and as an indicator electrode for calculating pH values in early studies.
What are the advantages of glass electrode?
The glass electrode is very sensitive, and it is used by many forms of smartphones. Cleaning and calibration using a common buffer solution is easy and simple to use. It covers an acidic and an alkaline pH spectrum.
What is the standard hydrogen electrode?
Normal hydrogen electrode: This is a reference electrode to which all electrodes are calculated in terms of electrode potential. If hydrogen gas is adsorbed at 1 atm-pressure over a platinum electrode dipped at 25oC in 1 M HCl, it is a regular electrode of hydrogen and its potential is E0=±0 volt.
Why is a platinum electrode used?
Platinum is an inert catalyst, because it does not engage in the reaction of the cells but provides an oxidation and reduction reaction surface or base. Platinum is used because hydrogen can quickly be adsorbed as well as inert metal does not participate in redox reaction during cell operation.
Which foil is used in standard hydrogen electrode?
SHE consists of a platinum electrode with a hydrogen ion concentration of 1.00M submerged in a solution. The platinum electrode consists of a tiny platinum foil square that is platinized. Hydrogen gas bubbles around the platinum electrode at a pressure of 1 atmosphere.

I want to know about its uses and limitations
its is used to as a reference on all half-cell potential reactions.