Teflon is a name we have been used to hearing from our childhood as it is an important element used in non-stick cookware utensils. Teflon is actually made of a chemical compound named polytetrafluoroethylene (PTFE) which is a synthetic fluoropolymer which has been under use for various purposes. The discoverer of PTFE named Roy Plunkett found the same while working for DuPont in New Jersey in 1938 by accident.
In the 1990s, it was found that PTFE could be radiation cross-linked above its melting point in an oxygen-free environment. Another example of radiation processing is electron beam processing. There is stability for radiation and better mechanical properties at high temperatures. This was significant because, for many years, irradiation under ambient conditions has been used to break down Polytetrafluoroethylene for recycling.
Table of Contents
How Teflon is Produced
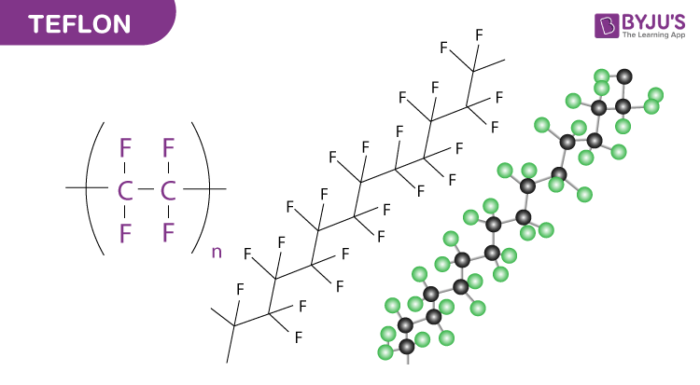
The chemical compound is produced by tetrafluoroethylene by free radical polymerization. The equation for the process is
n F2C=CF2 → −(F2C−CF2)n−
Because tetrafluoroethylene can explosively decompose to tetrafluoromethane and carbon, special apparatus is required for the polymerization to prevent hot spots that might initiate this dangerous side reaction. The process starts with the hemolyzing of persulphate that forms sulphate radicals:
[O3SO−OSO3]2− ⇌ 2 SO4•−
The resultant of this reaction is terminated using sulphate ester groups that could create OH end-groups by hydrolysing.
The polymerization is carried out as an emulsion in water because PTFE is weakly soluble in practically all solvents. Particles of polymer in the form of suspension are created by this process. Sometimes surfactants like PFOS are used for polymerization.
Chemical Properties
The compound is used as the coating for non-stick pans as they are hydrophobic and have a high resistance capacity to heat. Thermoplastic polymer PTFE has a melting point of 600 K (327 °C; 620 °F), is a white solid at ambient temperature, and has an approximate density of 2200 kg/m^3.
From the above discussion, it is evident why this chemical compound has fascinated scientists and students of science around the world and would continue to do so in the years to come.

i am using for study from 1month and because of this app imporved in my study