What is an Atom?
“Atoms are the building blocks of our world. Previously, it was assumed that atoms were like small balls, but definitions have changed. The atom is composed of three major particles: protons, neutrons, and electrons – which are made up of even smaller particles like quarks.”
An atom is the smallest particle of an element that may or may not have an independent existence but is always present in a chemical reaction. An atom is the smallest unit that retains an element’s properties. An atom is made up of subatomic particles, which cannot be created or destroyed. Atoms of the same element are all identical, whereas atoms of different elements are of different types. When atoms are rearranged, chemical reactions occur.
Table of Contents
- Creation of Atoms
- History of Atoms
- Atomic Models
- What Does an Atom Look Like Under a Microscope?
- Frequently Asked Questions – FAQs
Creation of Atoms
After the Big Bang 13.7 billion years ago, atoms were formed. As the hot, dense new universe cooled, conditions became favourable for the formation of quarks and electrons. Quarks combine to form protons and neutrons, which then combine to form nuclei. According to CERN (The European Organization for Nuclear Research), all of this occurred within the first few minutes of the universe’s existence.
It took 380,000 years for the universe to cool enough for the electrons to slow down enough for the nuclei to capture them and form the first atoms. The first atoms were mostly hydrogen and helium.
History of Atoms
The matter is made up of indivisible building blocks. Leucippus and Democritus recorded this concept as early as the fifth century BCE. The Greeks referred to these particles as atoms, which means indivisible, and the modern term “atom” is derived from this word. Democritus proposed that the various forms of matter were caused by different types and combinations of these particles. However, because most philosophers favoured the Aristotelian viewpoint at the time, these ideas were largely ignored.
Many scientists and philosophers, including Galileo, Newton, Boyle, and Lavoisier, re-evaluated and expanded on the concept of the atom.
Atomic Models
Throughout the history of atomic physics, there have been many different atomic models. Although the existence of atoms has been known for a very long time now. This article will focus on four basic atomic models:
- Dalton’s Billiard Ball Model
- J.J Thomson’s “plum pudding” model
- Rutherford’s Planetary model
- Bohr’s Atomic model
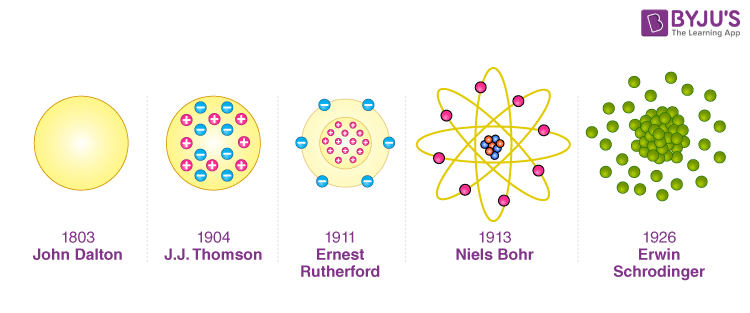
Dalton’s Billiard Ball Model
In 1803, an English chemist named John Dalton introduced his atomic model. Dalton’s atomic theory stated that atoms were invisible, unchangeable, indestructible building blocks of matter and that the weight of an element’s atoms determines its characteristics.
He stated that all compounds are merely combinations of atoms and that different elements have different atoms, much like Democritus’ model. Because of the striking resemblance between his hypothesised atom and a billiard ball, Dalton’s model was termed “the billiard ball model.”
Demerits of Dalton’s Atomic Theory
- Subatomic particles make up atoms.
- The assumption that all atoms have the same mass is incorrect as well as masses of atoms in various elements are different is flawed.
- There are allotropes forms of the same elements.
J. J. Thomson’s Model
J. J. Thomson proposed the “plum pudding model” in 1904. Atoms were made up of negatively charged electrons in this model, but the atomic nucleus had not yet been discovered. Thomson recognised that an atom’s total charge was neutral. He believed that there had to be something to balance an electron’s negative charge.
He proposed that the tiny negatively charged particles were embedded in the positively charged cloud-like seeds in a watermelon or like the plums in a pudding. Because of his resemblance to a popular English dessert, his model is known as the plum pudding model.

Failure of Model
The scientist was unable to provide experimental support for his atomic model.
The scientist was unable to explain the results of Rutherford’s and other scientists’ experiments later.
Rutherford Model
- The nucleus is introduced to atomic theory by the Rutherford model.
- Each atom has a nucleus, which is a positively charged centre. The nucleus contains nearly all of an atom’s mass.
- The electrons go in a circular path around the nucleus.
- In comparison to the size of the atom, the nucleus is quite small.
His atomic model is referred to as the planetary model. However, there were some significant flaws in this concept. For example, it cannot explain the stability of an atom.
Bohr’s Model
- Rutherford’s concept, in which electrons travel around in fixed orbital shells, was revised by Bohr. According to the Bohr atomic model-
- Negatively charged electrons surround a small positively charged nucleus in fixed orbits.
- He concluded that electrons occupy stable orbits or shells. Each orbital shell’s energy levels are fixed.
- The different energy levels or orbits are depicted in one of two ways: 1, 2, 3, 4… shells or K, L, M, N….. shells.
 Limitations
Limitations
It could not explain the Zeeman Effect, the Stark effect, Heisenberg Uncertainty Principle, and the spectra observed from bigger atoms.
What Does an Atom Look Like Under a Microscope?
Atoms are invisible to optical microscopes because they do not interact with light particles, resulting in no deflection. We didn’t get our first glimpse of the atom until the invention of electron microscopes.
When an electron beam with a shorter wavelength than visible light strikes a target, it scatters, allowing an image to be created. There are many more advanced microscopes available that allow us to observe atoms and help us move atoms around in a sample to study them.

Frequently Asked Questions on Atom
What is an atom?
Atoms are the building blocks of all materials. Thus, atoms are like the building blocks of everything and can combine to make new substances. One of the most common atoms is hydrogen (H).
Is it possible to see an atom?
Atoms are like extremely small bricks that can be used to construct any material. An atom is too small for the human eye to see.
What do atoms consist of?
Atoms are made up of two elementary particles: electrons and quarks. Electrons occupy the space that surrounds the nucleus of an atom. Each electron has a negative electrical charge. Quarks combine to form protons and neutrons, which form the nucleus of an atom.
How do atoms exit?
Most elements’ atoms exist as molecules or ions because they are the most reactive. For example, hydrogen, oxygen, chlorine, and so on. However, non-reactive atoms of some elements exist in nature in a free state. Helium, neon, argon, and other noble gases are examples.
What is the most widely accepted model of the atom?
Niels Bohr’s atom model is the most widely accepted. In 1913, Bohr’s model was first presented.
Comments