What is Zinc nitrate?
Zn(NO3)2 is an inorganic chemical compound with the chemical name Zinc nitrate. It is also called zinc dinitrate or celloxan or zinc nitrate Hexahydrate. It is widely used as a catalyst in the manufacture of medicine, dyes, and various other chemicals.
Celloxan is a white to colourless, crystalline solid which is highly deliquescent. It exists as hexahydrate i.e, Zn(NO3)2•6H2O. It is soluble in alcohol as well as water. It is a non-combustible compound but has the power to accelerate the burning of other combustible compounds. Toxic oxides of nitrogen are liberated when heated.
Properties of Zinc nitrate – Zn(NO3)2
| Zn(NO3)2 | Zinc nitrate |
| Molecular weight of Zn(NO3)2 | 189.36 g/mol (anhydrous) |
| Density of Zinc nitrate | 2.065 g/cm3 (anhydrous) |
| Melting point of Zinc nitrate | 110 °C |
| Boiling point of Zinc nitrate | Approximately 125 °C |
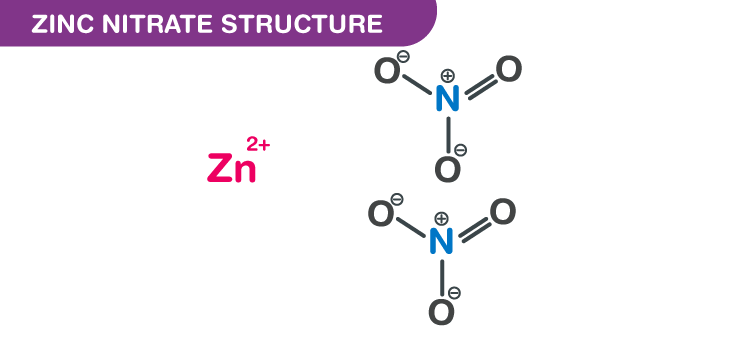
Zinc nitrate structure – Zn(NO3)2

Zn(NO3)2 Uses (Zinc nitrate)
- Zinc nitrate is used in the synthesis of coordination polymers.
- Used in dyeing as a mordant.
- Used as a catalyst in resin production.
- Used as a strong oxidizing agent.
- Used in liquid fertilizers.
- Used as a latex coagulant.
- Used in the manufacturing of medicines.
Production of Zinc nitrate
It is produced by dissolving zinc in nitric acid. The reaction is concentration-dependent, and forms ammonium nitrate. The reaction is given below:
Zn + 2 HNO3 (diluted) → Zn(NO3)2 + H2
4 Zn + 10 HNO3 (concentrated) → 4 Zn(NO3)2 + NH4NO3 + 3 H2O
It undergoes thermal decomposition on heating and forms zinc oxide, oxygen, and nitrogen dioxide.
2 Zn(NO3)2 → 2 ZnO + 4 NO2 + O2
Health hazards
Inhaling zinc nitrate dust causes irritation in the throat and nose. Swallowing Zinc dinitrate can lead to corrosion of the alimentary tract. Contact with the skin results in irritation and can cause rashes.
When heated, it may liberate toxic oxides of nitrogen. When it comes in contact with combustible material it can increase the fire intensity.
Frequently Asked Questions
What is zinc nitrate used for?
Zinc nitrate does not have a broad scale use, but is used for the synthesis of coordinating polymers on a laboratory scale. The controlled decomposition to zinc oxide can also be used for the generation of various structures, including nanowires. It can also be used as a dyeing mordant.
Is zinc nitrate acidic or basic?
Zinc nitrate is a salt of a weak base and strong acid. Zinc nitrate ionises to give zinc and nitrate ions in water. The basic radical Zn2+ hydrolysis to give weak base Zn(OH)2 and H+ ions are released which makes the solution acidic.
Does nitric acid react with zinc?
Aluminium and zinc do not react with concentrated nitric acid due to the formation of a dense, hard-to-dissolve oxidation layer due to a process known as passive oxidation. This protects the metal against further reaction and corrosion. Nevertheless, zinc does react with dilute nitric acid.
Learn more about the structure, physical and chemical properties of Zn(NO3)2 from the experts at BYJU’S.

Comments