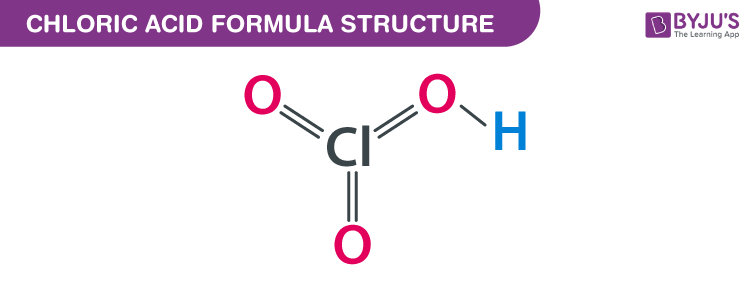
Chloric Acid formula, also known as Chloric (V) Acid formula or Hydrogenchloric Acid formula is explained in this article. It is an oxoacid of chlorine. It consists of one chlorine atom, one hydrogen atom, and three oxygen atoms. Two oxygen atoms attached to the chlorine atom are double bonded and the third atom is attached with a single bond. The chemical or molecular formula of Chloric Acid is HClO3.
Chloric Acid is a colourless liquid. It is a strong oxidizing agent and a strong acid. When it comes in contact with combustible materials it enhances the burning to ignite most. It is widely used as a reagent in chemical analysis and in the making of various chemicals.
Chloric Acid Formula Structure
Properties Of Chloric Acid
| Chemical formula | HClO3 |
| Molecular weight | 84.45914 g/mol |
| Density | 1 g/mL |
| pKa | Approximately −1 |
| Appears as | colourless solution |
Synthesising Method Of Chloric Acid
- Hydrogenchloric acid can be prepared by reacting sulfuric acid with barium chlorate and the insoluble barium sulfate is removed by precipitation.
- Another method of synthesizing chloric acid is by heating of hypochlorous acid to produce chloric acid along with hydrogen chloride.
.
To learn more about Chloric Acid formula from the expert faculties at BYJU’S, register now!
Comments