A large element of chance is inherited in the natural processes. For example, the spacing between trees is a random natural process. Likewise, falling of tree leaves on the ground with the random arrangement is also a random process. Entropy is the measure of disorders or randomness of the particular system. Since it depends on the initial and final state of the system, the absolute value of entropy cannot be determined. You need to consider the difference between the initial and final state to determine the change in entropy.
The change in Entropy Formula is expressed as
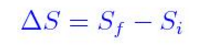
According to the thermodynamic definition, entropy is based on change in entropy (ds) during physical or chemical changes and expressed as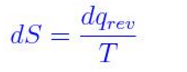
For change to be measurable between initial and final state, the integrated expression is
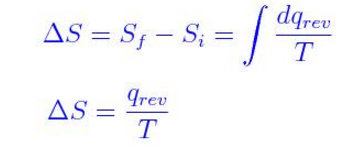
The units for entropy is calories per degree or Cal deg-1. Molar Entropy is written in joules per kelvin per mole (JK-1mol-1)
Solved Examples
Determine ΔS for the synthesis of ammonia at 25oc.
Consider Haber Process on Ammonia synthesis
N2 + 3H2 —> 2NH3
ΔH = -92.6kJ/mol
Solution
We can use the formula
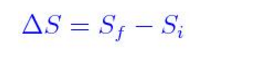
ΔS = 2(NH3) – [(N2) + 3(H2)]
ΔS = (2)(192.5 JK-1mol-1) – [191.6JK-1mol-1 + (3)(130.6 JK-1mol-1)]
ΔS = -198.4 JK-1mol-1
Comments