Hypobromous Acid is a weak and unstable acid which has a similar chemical and physical property as of hypohalites. hypohalites are the oxyanion having halogen in oxidation state +1. Hypobromous Acid has the capacity to kill pathogens and bacterias hence it is used as a disinfectant, oxidizer, bleach, and a deodorant.
Let us learn about the chemical properties of Hypobromous Acid.
| Chemical formula | HBrO or BrHO |
| Molecular weight | 96.911 g/mol |
| Density | 2.470 g/cm3 |
| Chemical names | Oxayl bromide, bromic(I) acid, bromanol, hydroxidobromine |
| Acidity (pKa) | 8.65 |
| Boiling point | 20–25 °C |
Hypobromous Acid Structural Formula
When bromine reacts with water hypobromous acid is formed.
Br2 + H2O ⇄ HOBr + HBr
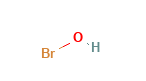
The structural formula of hypobromous acid is as shown in the figure below.
Use Of Hypobromous Acid
- Hypobromous acid is used as a deodorant, oxidizer and bleach.
Stay tuned with BYJU’S for more scientific information about chemical components!!
Comments