Hyposulfurous acid is an unstable oxoacid of sulfur and is found in oxidation state between dithionous acid and hydrogen sulfide. Hyposulfurous acid is an isomer of sulfinic acid and has no independent occurrence and is not been detected in the aqueous state. Let us know the chemical composition and other details of hyposulfurous acid.
Properties Of Hyposulfurous Acid
| Chemical formula | S(OH)2 |
| Molecular weight | 66.07 g·mol−1 |
| Chemical Names | Sulfur dihydroxide, Sulfoxylic acid,
Dihydroxidosulfur, sulfanediol |
| Conjugated base | Bisulfoxylate (SO2H−) |
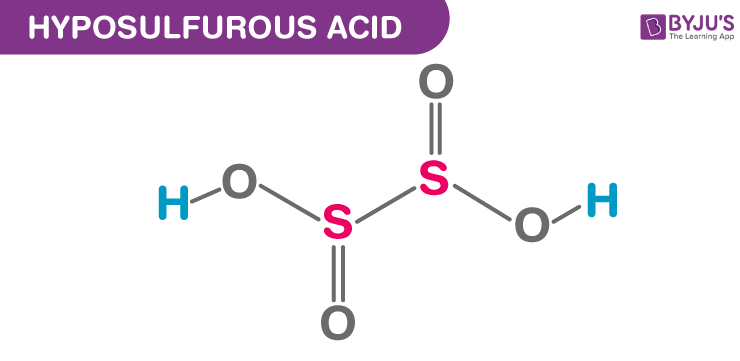
Hyposulfurous Acid Structural Formula
The structural representation of hyposulfurous acid is shown in the figure below. 2 hydroxy groups are attached to a sulfur atom. hyposulfurous acid includes sulfur in an oxidation state of +2.
For more information on any chemical compound, refer BYJU’S.
Comments