The Class 8 ICSE students are provided with comprehensive study material and Selina solutions for Chemistry prepared by experienced academic experts at BYJU’S. Going through the Selina Solutions provided by BYJU’S enhances your ability to write the answer to the point.
Let us now look at what the Chapter- Hydrogen deals with.
The first and foremost element of the periodic table, Hydrogen, is the lightest element known till date. The atomic number of hydrogen is 1.
Constituting almost 75% of the mass of the Universe, H1 or the monatomic form of hydrogen is the most abundant chemical substance in the Universe. Displacement reaction of metals forms the Hydrogen. Hydrogen is made by the reaction of sulphuric acid on zinc in a laboratory.
This chapter deals with everything related to hydrogen, including its action on litmus, combustibility, its action with different substances like oxygen, chlorine gas, metallic oxide, and finally its uses and tests.
Download ICSE Class 8 Chemistry Selina Solutions PDF for Chapter 7 – Hydrogen
Question 1
Fill in the blanks:
(a) Hydrogen is lighter than air.
(b) Hydrogen is sparingly soluble in water.
(c) Hydrogen bums with a oxyhydrogen pale blue flame and pop sound are heard.
(d) A metal sodium hydrogen in the reactivity series gives hydrogen with water.
(e) Hydrogen reacts with metal oxides to form metal and water.
(f) Oxidation is the removal of hydrogen and the addition of oxygen.
(g) In redox reaction oxidation and reduction occur simultaneously.
Question 2
Indicate which of the following statements are true and which are false:
(a) Hydrogen molecule is monovalent.
Solution: False
(b) The removal of hydrogen from a substance is called reduction.
Solution: True
(c) Nitric acid cannot be used to prepare hydrogen by its action on active metals?
Solution: False
(d) The reaction between hydrogen and nitrogen to form ammonia is reversible.
Solution: True
(e) Zinc can liberate hydrogen from water, acid and alkali solution.
Solution: True
(f) Hydrogen is combustible as well as a supporter of combustion.
Solution: False
(g) Hydrogen gas is easily liquefiable.
Solution: False
Question 3
Complete and balance the following equations:
(a) H2+_________⟶2HCl
(b) H2+S⟶_________
(c) Zn+_________⟶ZnCl2+H2
(d) CuO+_________⟶Cu+_________
(e) Fe + H2O⟶_________+________
(f) K +H2O⟶_________+________
Solution:
(a) H2+Cl2⟶2HCl
(b) H2+S⟶H2S
(c) Zn+2HCl⟶ZnCl2+H2
(d) CuO+H2⟶Cu+H2O
(e) 3Fe+4H2O⟶Fe3O4+4H2
(f) 2K+2H2O⟶2KOH+H2
Question 4
Give reasons for the following:
(a) Hydrogen be used as a fuel?
Solution:
Hydrogen is used as a fuel because it has a high heat of combustion. Some significant fuels are coal gas, water gas and liquid hydrogen.
(b) Though hydrogen is lighter than air it cannot be collected by downward displacement of air.
Solution:
Hydrogen is lighter than air so it is possible to collect the gas by downward displacement of air. But it is not safe to do so since a mixture of hydrogen and air can lead to an explosion.
(c) A pop sound produced when hydrogen is burnt?
Solution:
Impure hydrogen gas burns in air with a pop sound. This is because of the presence of impurities in it.
(d) Helium replaced hydrogen in weather observation balloons?
Solution:
It forms a mixture with air that can explode when there is a small leakage of hydrogen in a balloon. So helium has replaced hydrogen.
(e) Nitric acid not used for the preparation of hydrogen gas?
Solution:
(e) By the action of nitric acid on metals, hydrogen cannot be produced because it also releases nitrous oxide and nitric oxide and oxides the hydrogen to form water.
Question 5
Name the following:
(a) Two metals which give hydrogen with cold water.
Solution: Sodium (Na) and Potassium (K) give hydrogen with cold water.
(b) A metal which liberates hydrogen only when steam is passed over red hot metal.
Solution: Iron
(c) The process in which oxygen is added or hydrogen is removed.
Solution: Oxidation
(d) A metallic oxide which can be reduced into metal by hydrogen.
Solution: Copper oxide (CuO)
Question 6
(a) Name the chemicals required to prepare hydrogen gas in the laboratory.
Solution: Granulated Zinc and dil. Hydrochloric acid.
(b) Give a balanced chemical equation for the reaction.
Solution: Zn+2HCl→ZnCl2+H2 (g)
(c) Draw a neat and well-labelled diagram for the laboratory preparation of hydrogen.
Solution:
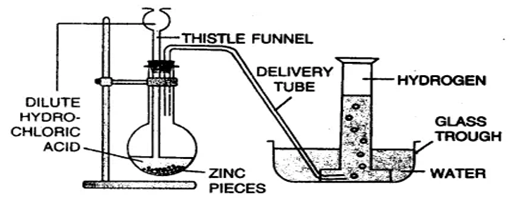
Preparation of hydrogen
(d) How is hydrogen gas collected?
Solution:
Hydrogen gas is collected by the downward displacement of water.
Question 7
How would you show that hydrogen:
(a) is a non-supporter of combustion?
(b) is lighter than air?
Solution:
(a) Hold a hydrogen gas-filled jar with its mouth downwards. Place a lighted candle inside the jar.
The gas burns with a pop sound and the candle gets extinguished. This shows that hydrogen is a non-supporter of combustion.
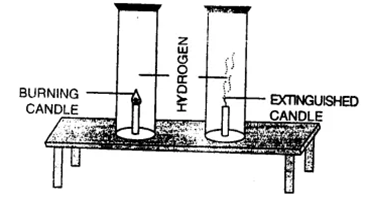
Hydrogen gas does not support combustion.
(b) Take a delivery tube and place one of its ends in a soap solution kept in a trough and the other one in a flat bottom jar as shown in the figure. The soap bubbles containing hydrogen rise upward the air. The rising soap bubbles prove that hydrogen is lighter than air.
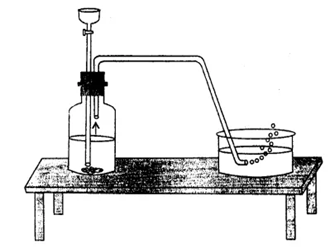
Hydrogen-filled soap bubbles rising upward in the soap solution and into the air shows that hydrogen is lighter than air.
Question 8
Hydrogen is a good reducing agent: What do you understand by the above statement? Explain with the help of copper oxide as an example.
Solution:
When hydrogen gas is passed over hot metallic oxides of copper, lead, iron, etc., hydrogen acts as a good reducing agent. It removes oxygen from them and thus reduces them to their corresponding metal.
For example, in each of which metallic oxide react with hydrogen. Metallic oxide act as oxidizing agents and hydrogen acts as a reducing agent.
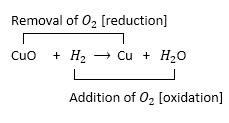
Question 9
(a) Name a process by which hydrogen gas is manufactured.
(b) Give equations for the reactions.
(c) How is hydrogen separated from carbon dioxide and carbon monoxide?
Solution:
(a) Commercially, hydrogen is prepared by Bosch process.
(b) (i) Steam is passed over hot coke at 1000°C in a furnace called converters. As a result water gas is produced which is a mixture of carbon monoxide and hydrogen gases.
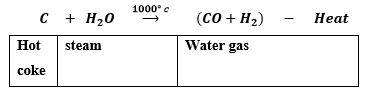
This reaction is endothermic in nature.
(ii) Water gas is mixed with excess of steam and passed over a catalyst ferric oxide and a promotor chromium trioxide
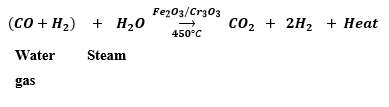
This reaction is exothermic in nature
(c) (i) Hydrogen, carbon dioxide are the products and some unreacted carbon monoxide. Hydrogen is separated from carbon dioxide by passing the mixture through water under pressure, in which carbon dioxide gets dissolved leaving behind hydrogen. Carbon dioxide can also be separated by passing it through caustic potash (KOH) solution.
2KOH+CO2→K2CO3+H2O
(ii) The gaseous mixture is passed through ammoniacal cuprous chloride to separate carbon monoxide in which carbon monoxide dissolves leaving behind hydrogen.
CuCl+CO+2H2O⟶CuCl•CO•2H2O
Thus hydrogen gas is obtained.
Question 10
Match the statements in Column A with those in Column B.
| Column A | Column B. |
| 1. A metal which reacts with cold water to form hydrogen | 1. Reduction. |
| 2. A gas which is inflammable and a non-supporter of combustion | 2. Hydrogenation |
| 3. A process in which vanaspati ghee is prepared from vegetable oils | 3. Oxidation |
| 4. The removal of hydrogen or addition of oxygen | 4. Sodium |
| 5. The addition of hydrogen or removal of oxygen | 5. Hydrogen |
Solution:
| Column A | Column B. |
| 1. A metal which reacts with cold water to form hydrogen | 4. Sodium |
| 2. A gas which is inflammable and a non-supporter of combustion | 5. Hydrogen |
| 3. A process in which vanaspati ghee is prepared from vegetable oils | 2. Hydrogenation |
| 4. The removal of hydrogen or addition of oxygen | 3. Oxidation |
| 5. The addition of hydrogen or removal of oxygen | 1. Reduction |
it was very useful