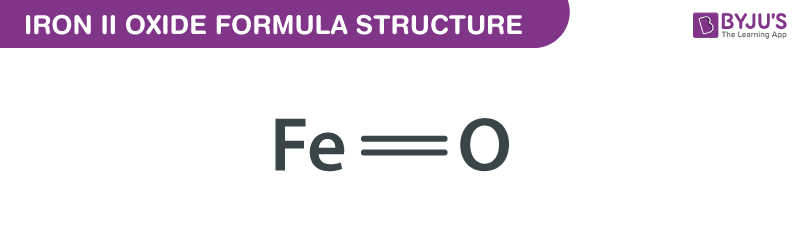
Iron II Oxide Formula formula, also known as Ferrous Oxide formula or Iron Monoxide formula is explained in this article. This inorganic compound consists of one iron atom and one oxygen atom. The chemical or molecular formula of Iron II Oxide Formula is FeO.
It occurs as a black crystalline solid. It does not dissolve in water and alkali. It is soluble in acids. Naturally, it occurs due to the incomplete oxidation of the metal. It is widely used as a raw material to manufacture steel, as a dye or pigment in the heat-absorbing glass in automobiles and buildings, and as a catalyst in the industrial operations.
Iron II Oxide Formula Structure
Properties Of Iron II Oxide
| Chemical formula | FeO |
| Molecular weight | 71.844 g/mol |
| Density | 5.745 g/cm3 |
| Boiling point | 3,414 °C |
| Melting point | 1,377 °C |
Uses Of Iron II Oxide
- Inhaling fumes or dust of iron (II) oxide can be hazardous and causes throat as well as nasal irritation.
- High levels of exposure may cause illness with flu-like symptoms, siderosis.
To learn more about Iron II Oxide Formula from the expert faculties at BYJU’S, register now!
Comments