Claisen condensation is one of the most important reactions of esters included in JEE syllabus. It is generally described as an ester analogy of aldol condensation.
Important Questions For Claisen Condensation
Some FAQs related to Claisen condensation are:
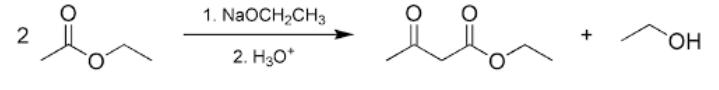
Question: What is Claisen condensation?
Answer: When molecules of ester containing at least one ?-hydrogen atom are exposed to alkoxide ions a new carbon-carbon bond is formed leading to the formation of ?-keto ester. This reaction is commonly known as Claisen condensation.
Questions: What is the general mechanism of Claisen condensation?
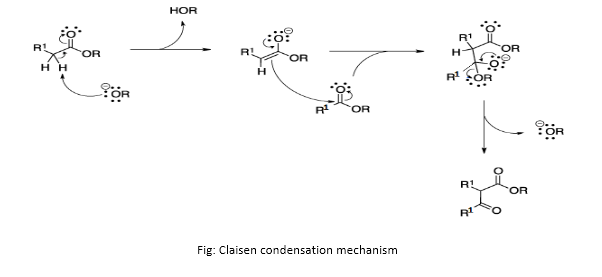
Answer: Mechanism of Claisen condensation is similar to the mechanism of aldol condensation except an ester is involved in place of aldehyde. An alkoxide is generally used as a base. A general mechanism is described below:
- The alkoxide ion acts as a base and removes the acidic ?-hydrogen atom from the ester molecule to form ester enolate. This reaction is an example of an acid-base reaction.
- Freshly formed ester enolate acts as a nucleophile and attacks the carbonyl carbon of the other ester molecule to form an intermediate ion. This reaction is an example of nucleophilic substitution reaction.
- Thus formed intermediate ion loses the alkoxide group stabilizing the ion to reform C=O. This finally leads to the formation of ?-keto ester.
Questions: Which reference books can one follow for Claisen condensation?
Answer: To develop a good understanding of Claisen condensation reaction, one needs to follow a reference book other than NCERT textbook for the mechanism of this reaction. Some authentic books for understanding the detailed mechanism of Claisen condensation include books from authors like Solomons & Fryhle, Morrison & Boyd. Once you have developed concepts on this topic, you can practice questions from the books of authors like M. S. Chauhan, etc. or from previous years JEE question bank.
For further details related to Claisen condensation for JEE chemistry, get in touch with our mentors here at BYJU’S.
Comments