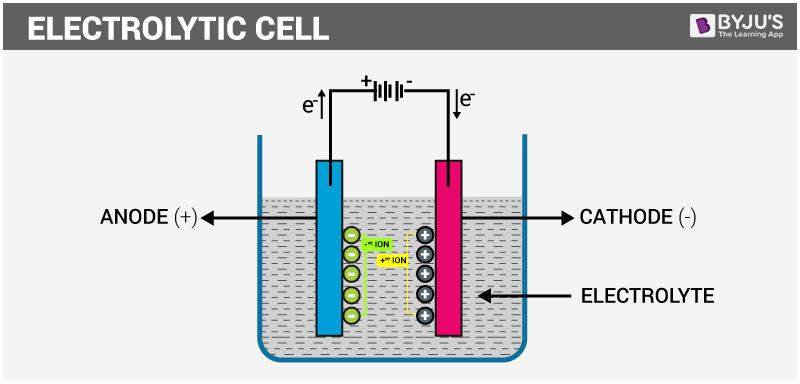
Electrochemistry is the branch of chemistry that deals with the chemical changes produced by electric current and the electricity generation by chemical changes. An electrochemical cell consists of 2 metallic conductors known as electrodes in contact with an ionic conductor i.e. an electrolyte. The electrolyte and the electrode comprise an Electrode Compartment. An Electrolytic Cells are those in which a non-spontaneous reaction is controlled by an external source of electric current. The Galvanic Cells generate electric current due to spontaneous cell reaction. In a galvanic cell, the cathode is + ve w.r.t. the anode whereas, in an electrolytic cell the anode is + ve w.r.t. the cathode. The decomposition of electrolyte solution by passing current, resulting in the liberation of gases or metal deposition at electrodes is termed as electrolysis. In an Electrolytic cell, the electrical energy is converted into chemical energy.
Important Questions For Electrochemistry
Q1: What are the important topics in electrochemistry?
Sol: Electrochemistry does fall under the important topics, which help you in scoring good in JEE. Following are the concepts that should be studied carefully:
- Electrolysis and electrolytic cell
- Daniel cell
- Electrochemical series
- Electrode Potential
- Arrhenius Theory of Electrolytic Dissociation
- Faraday’s law of Electrolysis
- Nernst equation
- Electrolytic Conduction
- Kohlrauschs Law
- Batteries
- Concentration cells
- Application of Electrolysis
Q2: Find the charge in coulomb (C) on 1 (g)gram-ion of N3-.
Sol:
Charge on one ion of N3-in Coulomb = 3 × 1.6 × 10-19C
Thus, charge on 1 gram (g) ion of N3-in Coulomb = 3 × 6.02 × 1023× 1.6 × 10-19= 2.89 × 105C.
Electrochemistry – Important Topics

Electrochemistry – Important Questions

Comments