Environmental chemistry is the study of chemical and biochemical phenomenon that occur in nature. It is not related to green chemistry which aims at reducing pollution. The frequently asked questions for JEE from this topic are as follows:
Important Questions For Environmental Chemistry
Question 1: What is environmental pollution?
Answer: Environment refers to the surroundings of an organism. It consists of both living and non-living components. Living components include animals, plants and micro-organisms. The non-living components are air, water, soil, atmosphere etc.
When there is an undesirable change in the surrounding that has harmful effects on plants and animals, it leads to environmental pollution. A pollutant is a substance that causes pollution. We can have pollutants in a liquid, solid or gaseous state. A substance becomes a pollutant when its concentration is greater than the natural abundance and this increase in concentration is either due to human activities or natural phenomenon.
Question 2: What is ozone? Give reactions for ozone formation.
Answer: Ozone layer is found in the upper regions of the stratosphere where it protects the earth from the harmful ultraviolet rays of the sun. These radiations can cause skin cancer in humans. The ultraviolet rays split the oxygen molecule into free oxygen atoms, these free oxygen atoms combine with the oxygen molecule to form ozone.
Ozone is unstable and decomposes to molecular oxygen. Therefore a dynamic equilibrium is maintained between the formation and decomposition of ozone.
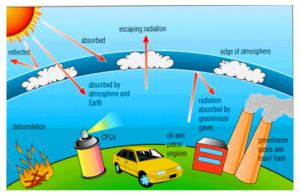
Answer: The ozone layer present in the stratosphere acts as a protective shield. It saves the earth from the harmful ultraviolet rays of the sun. Once the ozone layer is depleted, ultraviolet rays pass through the troposphere and eventually reach the earth’s surface. These rays cause ageing of the skin, skin cancer, cataract and sunburn to humans as well as other animals. Phytoplanktons die in the presence of ultraviolet rays which results in a decrease in fish productivity. Thus the quality of life on earth is hampered due to the depletion of the ozone layer.
For more information on this topic or JEE install BYJU’S – the learning app.
Environmental Chemistry

Comments