KVPY-SX 2020 Chemistry question paper is provided here to assist students in understanding the overall nature of the question paper that is set in the exam. Students can easily access the question paper as it can be directly viewed on the website or they can also download the KVPY SX 2020 Chemistry paper PDF and use it at a later instance.
In addition, each question in the KVPY 2020 Chemistry paper has been answered by our experts and the solutions given will further help them to learn and revise the important topics that have been covered. Students will also get a much better understanding of the types of questions, their difficulty level, weightage of marks and more. Ultimately, as students continue to practice solving the questions from different papers they will be able to prepare effectively, develop better problem solving skills and clear the upcoming examination.
Question 1: The stability of
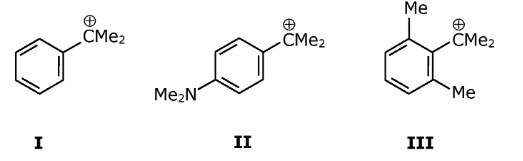
Follows the order
a. I > II > III
b. II > I > III
c. II > III > I
d. III > II > I
Answer: (b)
Stability of carbocation increases with (+M) effect
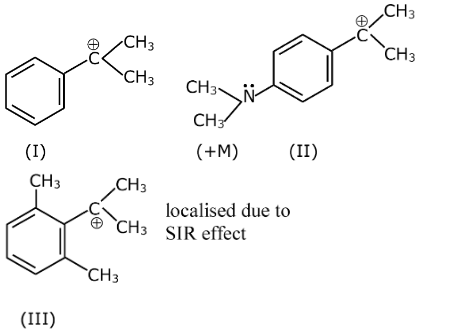
Structure-II has +M effect of –NMe2 group, hence maximum stability among the given set of cations. In structure-III due to steric inhibition of resonance effect –C+Me2 moves out of the plane of the benzene ring hence stability decreases due to loss of conjugation.
Finally, the correct order should be: II > I > III.
Question 2: Among the following, the biodegradable polymer is:
a. Polylactic acid
b. Polyvinyl chloride
c. Bakelite
d. Teflon
Answer: (a)
Polylactic acid (PLA) is a biodegradable aliphatic polyester, PLA sometimes called polylactide, which can be produced by fermentation of renewable resources such as corn, cassava, potato and sugarcane.
Question 3: Among the following,
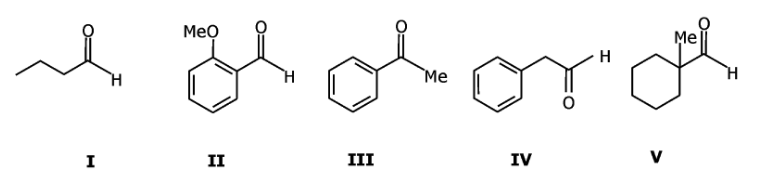
The compounds which can be reduced with formaldehyde and conc. aq. KOH are:
a. only II and V
b. only I and V
c. only II and III
d. only I, II and IV
Answer: (a)
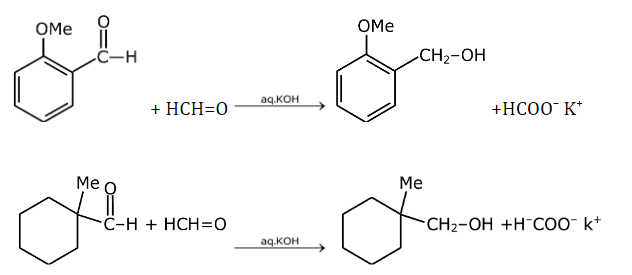
These are examples of Cannizzaro reactions.
Aldehyde without α-H gives a Cannizzaro reaction and forms alcohol and salt of carboxylic acid.
Question 4: An organic compound that is commonly used for sanitizing surfaces is:
a. acetylsalicylic acid
b. chloramphenicol
c. aspartame
d. cetyltrimethylammonium bromide
Answer: (d)
Cetyltrimethylammonium bromide is a popular cationic detergent. Cationic detergents have germicidal properties and are expensive.
Question 5: The rates of reaction of NaOH with
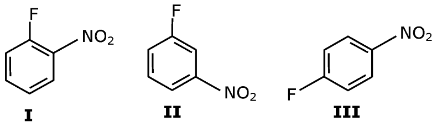
follow the order:
a. II > I > III
b. II > III >I
c. I > III > II
d. III > II > I
Answer: (c)
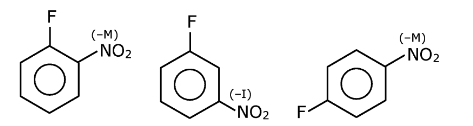
I and III can react with NaOH but II do not react to room temperature. I and III give a reaction because at ortho and para position electron-withdrawing group is present.
Question 6: The most suitable reagent for the conversion of 2-phenylpropanamide into 1-phenylethylamine is:
a. H2, Pd/C
b. Br2, NaOH
c. LiAlH4, Et2O
d. NaBH4, MeOH
Answer: (b)
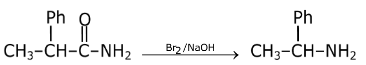
Hoffmann bromamide degradation reaction.
Question 7: The compound X in the following reaction scheme is:
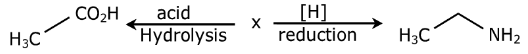
a. acetonitrile
b. methyl isocyanide
c. acetaldehyde
d. nitromethane
Answer: (a)
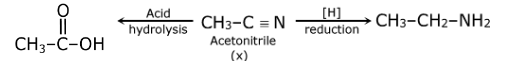
Question 8: A nucleus X captures a β particle and then emits a neutron and γ ray to form Y. X and Y are:
a. isomorphs
b. isotopes
c. isobars
d. isotones
Answer: (d)
zXA + –1e0 →Z–1YA → Z–1XA–1(Y) + 0n1 + γ
A = Mass number of X
Z = Atomic number of X
Number of neutrons in X = A – Z
Number of neutrons in Y = A – 1 – (Z – 1) = A – Z
i.e., X & Y are isotones (have the same number of neutrons)
Question 9: The boiling point (in 0C) of 0.1 molal aqueous solutions of CuSO4 · 5H2O at 1 bar is closest to:
[Given: Ebullioscopic (molal boiling point elevation) constant of water. Kb = 0.512 K Kg mol–1]
a. 100.36
b. 99.64
c. 100.10
d. 99.90
Answer: (c)
∆Tb = iKb .m
i = Van’t Hoff factor
CuSO4→ Cu+2 + SO4-2 (i = 2)
∆Tb = 2 × 0.512 × 0.1
∆Tb = 0.1024
Tsol– T0A = ∆Tb
Tsol – 100 = 0.1024
Tsol = 100.1024 0C
Question 10: A weak acid is titrated with a weak base. Consider the following statements regarding the pH of the solution at the equivalence point:
(i) pH depends on the concentration of acid and base.
(ii) pH is independent of the concentration of acid and base.
(iii) pH depends on the pKa of acid and pKb of the base.
(iv) pH is independent of the pKa of acid and pKb of the base.
The correct statements are:
a. only (i) and (iii)
b. only (i) and (iv)
c. only (ii) and (iii)
d. only (ii) and (iv)
Answer: (c)
For weak acid and weak base titration pH formula is
[H]+ = √(KwKa/Kb)Take -ve log
pH = +½ (pKw+ pKa – pKb)
pH = 7 +½ (pKa – pKb)
Question 11: Products are favoured in a chemical reaction taking place at a constant temperature and pressure.
Consider the following statements:
(i) The change in Gibbs energy for the reaction is negative.
(ii) The total change in Gibbs energy for the reaction and the surroundings is negative.
(iii) The change in entropy for the reaction is positive.
(iv) The total change in entropy for the reaction and the surroundings is positive.
The statements which are ALWAYS true are:
a. only (i) and (iii)
b. only (i) and (iv)
c. only (ii) and (iv)
d. only (ii) and (iii)
Answer: (b)
The reaction is taking place at constant temperature and pressure and products formation are favoured. i.e., the reaction is spontaneous.
ΔSTotal = ΔSsys+ΔSsurr> 0
At constant T & P
ΔGreaction< 0 (spontaneous)
Correct statements : (i) & (iv)
Question 12: A mixture of toluene and benzene forms a nearly ideal solution. Assume PB0 and PT0 to be the vapour pressures of pure benzene and toluene, respectively. The slope of the line obtained by plotting the total vapour pressure to the mole fraction of benzene is:
a. P0B – P0T
b. P0T – P0B
c. P0B + P0T
d. (P0B -P0T)/2
Answer: (a)
According to Raoult’s law
PS = PB + PT
PS = PB0xB + PT0xT
PS = PB0xB+ PT0(1-xB)
PS = (PB0 – P0T)xB + P0T
y = mx + c

Question 13: Upon dipping a copper rod, the aqueous solution of the salt that can turn blue is:
a. Ca(NO3)2
b. Mg(NO3)2
c. Zn(NO3)2
d. AgNO3
Answer: (d)
Cu + 2AgNO3→Cu(NO3)2 + 2Ag
Blue
Order of reactivity:Ca> Mg>Zn> Cu>Ag
Hence Ag+ alone can oxidise Cu to Cu2+.
Question 14: Treatment of alkaline KMnO4 solution with KI solution oxidizes iodide to:
a. I2
b. IO4–
c. IO3–
d. IO2–
Answer: (c)
2KMnO4 + H2O + KI → 2MnO2 + 2KOH + KIO3
Question 15: If an extra electron is added to the hypothetical molecule C2, this extra electron will occupy the molecular orbital:
a. π*2p
b. π2p
c. σ*2p
d. σ2p
Answer: (d)
Due to sp-mixing energy order of molecules have less than or equal to 14-proton is-
σ1s<σ*1s<σ2s<σ*2s<π2p = π2p<σ2p<π*2p = π*2p<σ*2p
Molecular orbital configuration of C2-molecule – σ1s2σ1s*σ22s σ2s*2 π2p2 = π2p2
C2→ C2– (π2p2 = π2p2 σ2p1)
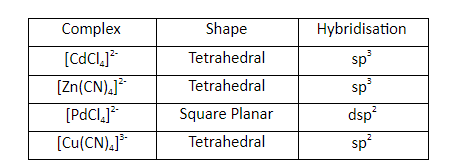
Question 16: Among the following, the square planar geometry is exhibited by
a. CdCl42–
b. Zn(CN)42–
c. PdCl42–
d. Cu(CN)43–
Answer: (c)
Pt+2 and Pd+2 – form a square planar and diamagnetic complex.
Question 17: The correct pair of orbitals involved in π-bonding between metal and CO in metal carbonyl complexes is:
a. metal dxy and carbonyl πx*
b. metal dxy and carbonyl πx
c. metal
d. metal
Answer: (a)
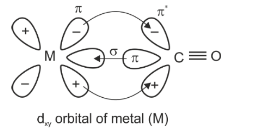
Synergic bonding interactions are present in metal carbonyls. The sigma bond is formed when a lone pair of electrons of carbonyl carbon is donated to the vacant orbital of metal and to form a π − bond, an electron pair is donated from fully filled metal dxy orbital to the vacant π* of CO. This involves the lateral overlap of orbitals.
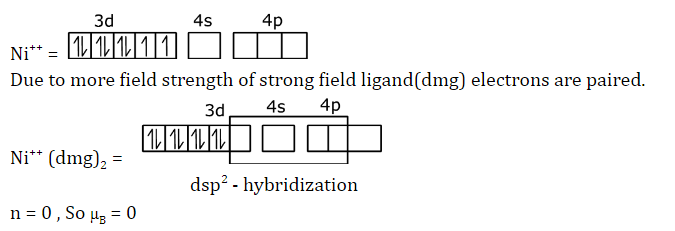
Question 18: The magnetic moment (in μB) of [Ni(dimethylglyoximate)2] complex is closest to:
a. 5.37
b. 0.00
c. 1.73
d. 2.25
Answer: (b)
μB = √(n(n+2))BM
n = no. of unpaired electron
Question 19: A compound is formed by two elements M and N. Element N forms a hexagonal closed packed array with 2/3 of the octahedral holes occupied by M. The formula of the compound is:
a. M4N3
b. M2N3
c. M3N2
d. M3N4
Answer: (b)
N → HCP (Z = 6)
The number of atoms in HCP is 6. It has six octahedral voids.
M → ⅔ ×O.V.
⇒ ⅔ ×6 ⇒ 4 (Z = 4)
Formula is
M4N6 ⇒ M2N3
Question 20: If the velocity of the revolving electron of He+ in the first orbit (n = 1) is v. the velocity of the electron in the second orbit is:
a. v
b. 0.5v
c. 2v
d. 0.25v
Answer: (b)
V = 2.18×106×(Z/n) m/sec
V ∝ 1/n
V1/V2 = n2/n1
V/V2 = 2/1
V2 = V/2 = 0.5V
Part II
Question 21: An organic compound X with molecular formula C11H14 gives an optically active compound on hydrogenation. Upon ozonolysis, X produces a mixture of compounds – P and Q. Compound P gives a yellow precipitate when treated with I2 and NaOH but does not reduce Tollens’ reagent. Compound Q does not give any yellow precipitate with I2 and NaOH but gives Fehling’s test. The compound X is:
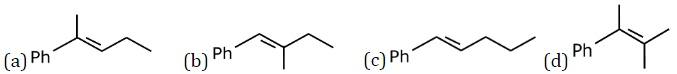
Answer: (a)
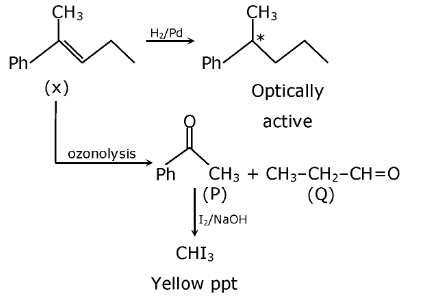
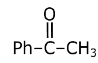
gives Iodoform which is yellow ppt but it does not give Tollen’s reagent test due to the presence of ketone group. CH3–CH2–CH=O does not give an iodoform reaction but can give Tollen’s reagent test.
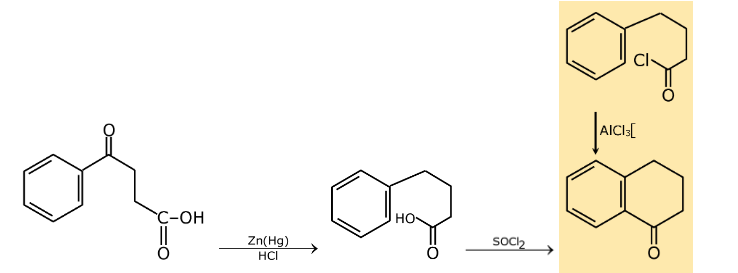
Question 22: The following transformation
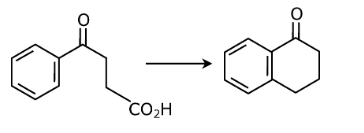
can be carried out in three steps. The reagents required for these three steps in their correct order, are:
a. (i) NaBH4; (ii) PCl5 ; (iii) anh. AlCl3
b. (i) NaBH4; (ii) anh. AlCl3; (iii) Zn(Hg)/HCl
c. (i) Zn(Hg)/HCl; (ii) SOCl2 ; (iii) Anh. AlCl3
d. (i) conc. H2SO4; (ii) H2N-NH2·H2O; (iii) KOH, ethylene glycol, Δ
Answer: (c)
Question 23: In the following reaction
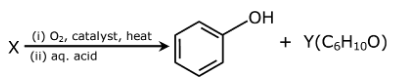
X and Y, respectively are :
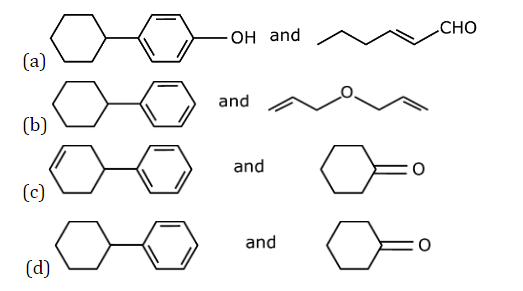
Answer: (d)
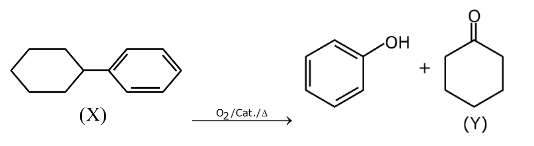
Cumene hydroperoxide oxidation reaction.
Question 24: A two-dimensional solid is made by alternating circles with radius a and b such that the sides of the circles’ touch. The packing fraction is defined as the ratio of the area under the circles to the area under the rectangle with sides of length x and y.
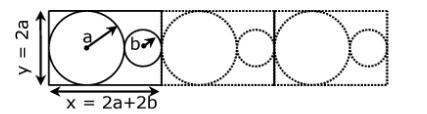
The ratio r = b/a for which the packing fraction is minimized is closest to:
a. 0.41
b. 1.0
c. 0.50
d. 0.32
Answer: (a)
Area of rectangular = xy
= 2a(2a + 2b)
Area covered by circles = πa2 + πb2
= π(a2+b2)
Packing fraction (PF) = (a2 +b2)/(2a+2b)(2a)
PF = πa2(1+(b/a))2/4a2(1+b/a) = π(1+r2)/4(1+r) ; [r = b/a]
d(PF)/dr = π/4 [(1+r)2r – (1+r2)/(1+r)2] = 0 if P.F. is minimum.
∴2r + 2r2 – 1 – r2 = 0
r2 + 2r -1 = 0
r = (-2±√(4+4))/2
= (-2+2√2)/2
= √2-1
≈ 0.41
Question 25: Consider a reaction that is first order in both directions
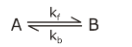
Initially only A is present, and its concentration is A0. Assume At and Aeq are the concentrations of A at time “t” and at equilibrium, respectively. The time “t” at which At = (A0 + Aeq)/2 is:
a. t = (ln(3/2))/ (kf+kb)
b. t = (ln (3/2))/(kf–kb)
c. t = (ln 2)/(kf+kb)
d. t = (ln 2)/(kf–kb)
Answer: (c)
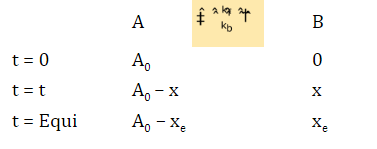
-d[A]/dt = kf[A] – kb[B]
-d[A]/dt = kf (A0 – xe) – kb(xe) = 0 [at equilibrium]
kf(A0 –xe) = kb(xe)
kb = kf (A0 – xe)/xe
+d[B]/dt = kf(A0 –x) – (kf(A0– xe)/xe) (x)
= xekf(A0-x) – kf(A0– xe)x/xe
+d[B]/dt = kfA0(xe-x)/xe
d[B]/(xe-x) = kf(A0/xe)dt
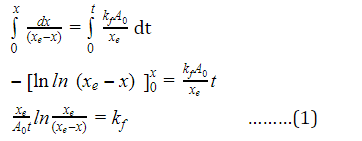
(xe/A0t) ln xe/(xe-x) = kf ………(1)
From old relation
Kb = kf(A0-xe)/xe
Kbxe = kfA0–kfxe
(kb + kf ) = kff A0/xe………..(2)
From equation (1) and (2)
(kf + kb) = (1/t) ln xe/(xe-x)
Now given data
⇒ (A0 – x) = At
(A0 – At) = x
⇒ At = (A0+ A0-xe)/2 = (2A0-xe)/2
2At = 2A0 – xe
xe = 2A0 – 2At
(xe – x) = 2A0 – 2At – A0 + At
(xe – x) = (A0 – At)
(kf + kb) = 1/t ln xe/(xe-x)
t = 1/(kf + kb) ln 2A0-2At/(A0-At)
= (1/(kf+kb)) ln2
Question 26: The reaction CaCO3(s) ⇌ CaO(s) + CO2 (g) is in equilibrium in a closed vessel at 298 K. The partial pressure (in atm) of CO2(g) in the reaction vessel is closest to:
[Given: the change in Gibbs energies of formation at 298 K and 1 bar for
CaO (s) = –603.501 kJ mol–1
CO2 (g) = –394.389 kJ mol–1
CaCO3 (s) = –1128.79 kJ mol–1
Gas constant R = 8.314 J K–1 mol–1]
a. 1.13 × 10–23
b. 0.95
c. 1.05
d. 8.79 × 1023
Answer: (a)
CaCO3(s) ⇌ CaO(s) + CO2 (↑)
ΔG0 = ∑∆G0product– ∑∆G0reactant
ΔG0= {ΔG0 [CaO(s)] + ΔG0 [CO2 (↑)]} – {ΔG0 [CaCO3(s)]}
ΔG0 = {–603.501 + (–394.389)] – [- 1128.79]
ΔG0= – 997.89 + 1128.79
ΔG0= 130.9 kJ/mole
ΔG0 = – 2.303 RT log Kp
130.9 = – 2.303 × 8.314 × 10–3 × 298 log KP
logKP = – 22.94
KP = Antilog (-22.94)
KP = 1.13 × 10–23
KP = PCO2= 1.13×10–23
Question 27: ∆A container is divided into two compartments by a removable partition as shown below:
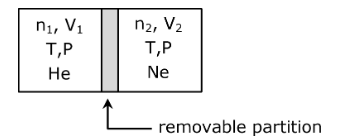
In the first compartment, n1 moles of ideal gas He is present in a volume V1. In the second compartment, n2 moles of ideal gas Ne is present in a volume V2. The temperature and pressure in both the compartments are T and P, respectively. Assuming R is the gas constant, the total change in entropy upon removing the partition when the gases mix irreversibly is:
a. n1 R ln (v1/(v1+v2)) + n2R ln (v1/(v1+v2))
b. n1R ln ((v1+v2)/v1) + n2R ln (v1+v2)/v1
c. (n1+n2) R ln n1v1/n2v2
d. (n1+n2)R ln n2v2/n1v1
Answer: (b)
Entropy is a state function
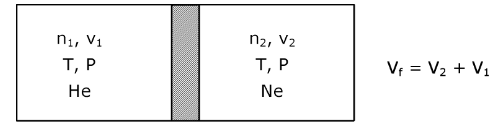
Entropy change ΔS = n CVln(Tf/Ti )+ nR ln(Vf/Vi)
For isothermal reversible process
∆S = nRln (Vf/Vi)
∆ST = ∆S1st compartment + ΔSIInd compartemnt
∆ST = n1Rln (V2+V1)/V1 + n2R ln(V2+V1)/V2
Question 28: Number of stereoisomers possible for the octahedral complexes [Co(NH3)3Cl3] and [Ni(en)2Cl2], respectively, are:
[en = 1,2-ethylenediamine]
a. 2 and 4
b. 4 and 3
c. 3 and 2
d. 2 and 3
Answer: (d)
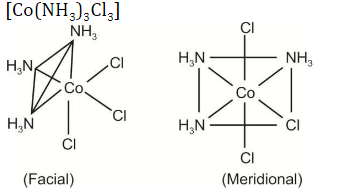
The possible number of stereoisomers of the above compound is 2.
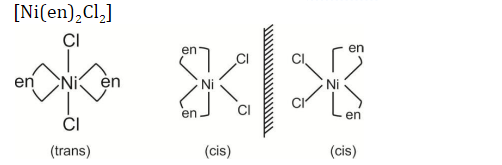
The possible number of stereoisomers of the above compound is 3.
Question 29: When a mixture of NaCl, K2Cr2O7 and conc. H2SO4 is heated in a dry test tube, a red vapour (X) is evolved. This vapour (X) turns an aqueous solution of NaOH yellow due to the formation of Y.
X and Y, respectively, are:
a. CrCl3 and Na2Cr2O7
b. CrCl3 and Na2CrO4
c. CrO2Cl2 and Na2CrO4
d. Cr2(SO4)3 and Na2Cr2O7
Answer: (c)
This is a chromyl chloride test of Cl– ion
4NaCl + K2Cr2O7 + 6H2SO4 → 2CrO2Cl2(X) + 2KHSO4 + 6H2O + 4NaHSO4
CrO2Cl2 + 4NaOH → Na2CrO4(Y) + 2NaCl + 2H2O
X = CrO2Cl2
Y = Na2CrO4
Question 30: Sodium borohydride upon treatment with iodine produces a Lewis acid (X), which on heating with ammonia produces a cyclic compound (Y) and a colorless gas (Z). X, Y and Z are:
a. X = BH3; Y = BH3·NH3; Z = N2
b. X = B2H6; Y = B3N3H6; Z = H2
c. X = B2B6; Y = B6H6: Z = H2
d. X = B2H6: Y = B3N3H6: Z = N2
Answer: (b)
2Na[BH4] + I2→ B2H6(X) + 2NaI + H2
X = B2H6
Y = B3N3H6
Z = H2
Comments