What Is Hydrogen Peroxide (H2O2)?
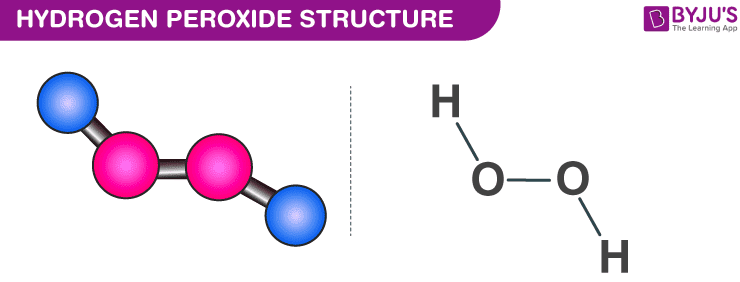
Hydrogen peroxide is the simplest kind of peroxide available (oxygen-oxygen single bond). It is a colourless liquid and is used in aqueous solution for safety reasons. It acts as a bleaching agent and is also used as a disinfectant. Concentrated hydrogen peroxide is a very reactive oxygen species and is used as a propellant in rocketry. The chemical formula for hydrogen peroxide is H2O2.
Table of Contents
- Overview
- Structure of Hydrogen Peroxide
- Preparation of H2O2
- Hydrogen Peroxide Properties
- Concentration of H2O2
Hydrogen peroxide is often referred to as water with one more oxygen atom. It is acidic in nature, and PH is about 4.5. It is a 100 per cent degradable compound.
Overview of H2O2 – Hydrogen Peroxide
| Hydrogen Peroxide Chemical Formula | H2O2 |
| Molecular Weight/Molar Mass | 4.0147 g/mol |
| Density | 1.05 g/cm3 |
| Boiling Point | 150.2 °C |
| Melting Point | -0.43 °C |
Structure of Hydrogen Peroxide
The structure of hydrogen peroxide is non-planar. H2O2 has an open-book structure with O–O spins. The dihedral angle is 111°. The O-O bond length is 145.8 pm, and the O-H bond length is 98.8 pm (which is equal to 9.88 × 10-13 m). The following diagram clearly shows what an open book structure means.
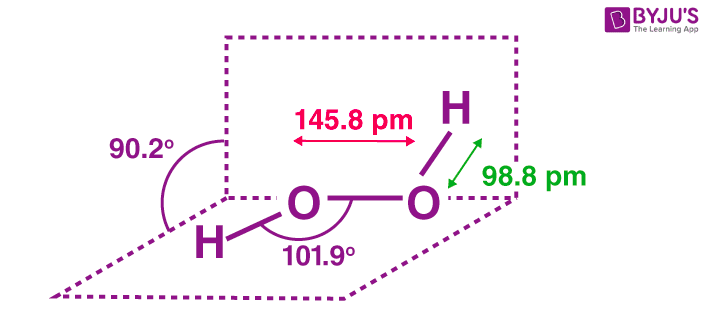
Hydrogen Peroxide Structure
There are two planes in this structure, and each plane has one O-H bond pair, the angle between both planes is 90.2°.
⇒ Also Read: Chemical Bonding
Preparation of Hydrogen Peroxide
Let us have a look at the various methods of preparation for hydrogen peroxide.
Laboratory Methods of Preparation
When barium peroxide is acidified, and the excess water is removed by the process of evaporation under reduced pressure, we obtain hydrogen peroxide. The following reaction will clarify this:
BaO2.8H2O(s) + H2SO4(aq) → BaSO4(s) + H2O2(aq) + 8H2O(l)
Industrial Method of Preparation
Hydrogen peroxide is prepared by the electrolysis of 30% ice-cold H2SO4. When acidified sulfate solution is electrolysed at a high current density, peroxodisulphate is obtained. Peroxodisulphate is then hydrolysed to get hydrogen peroxide.
2HSO–4(aq) [Electrolysis] → HO3SOOSO3H(aq) [Hydrolysis] → 2HSO–4(aq)+2H+(aq)+H2O2(aq)
Reaction Mechanism
- Electrolyte: 30% dilute H2SO4
- Cathode: Pb wire
- Anode: Pt rod
2H2SO4 → 2H+ + 2HSO–4
At Cathode: 2H+ + 2e– → H2
At Anode:
2HSO–4 → H2S2O8 + 2e– ⇒ Peroxodi Sulphuric Acid [Marshall’s acid]
H2S2O8 + H2O → H2SO5 + H2SO4 ⇒ Peroxomono Sulphuric Acid [Caro’s acid]
H2SO5 + H2O → H2SO4 + H2O2
Commercial Methods of Preparation
Auto-Oxidation Method:
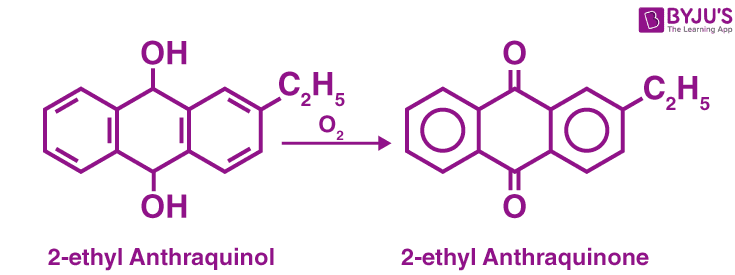
Auto-Oxidation Method of Preparation of H2O2
Properties of Hydrogen Peroxide
Physical Properties
- In the pure state, hydrogen peroxide is an almost colourless (very pale blue) liquid.
- It melts at 272.4 K and has a boiling point of 423 K (extrapolated).
- It is miscible in water in all proportions and forms hydrates.
Chemical Properties
Hydrogen peroxide in both acidic and basic mediums acts as an oxidising as well as a reducing agent. The following reactions will give a clear picture:
Oxidation Reactions of H2O2
- Oxidises black Pbs to white PbSO4. Reaction: Pbs + 4H2O2 → PbSO4 + 4H2O
- Oxidises KI to Iodine. Reaction: 2KI + H2O2 → 2KOH + I2
- Oxidises nitrites to nitrates. Reaction: NaNO2 + H2O2 → NaNO3 + H2O
- Oxidises acidified potassium ferrocyanide. Reaction: 2K4Fe(CN)6 + H2SO4 + H2O2 → 2K3Fe(CN)6 + K2SO4 + 2H2O
- Oxidises sulphites to sulphates. Reaction: Na2SO3 + H2O2 → Na2SO4 + H2O
- Oxidises CrO-24 in an acid medium to chromium peroxide CrO5. Reaction: CrO-24 + 2H+ + 2H2O2 → CrO5 + 3H2O
⇒ Check: Redox Reactions
Bleaching Reactions
It bleaches by oxidation. H2O2 bleaches silk, wool, cotton and hair.
Reaction: H2O2 → H2O + [O] Nascent oxygen
Coloured substance + [O] → colourless
Reduction Reactions of Hydrogen Peroxide
- It reduces barium peroxide to monoxide. Reaction: BaO2 + H2O2 → BaO + H2O + O2
- It reduces ozone to oxygen. Reaction: O3 + H2O2 → H2O + 2O2
- It reduces alkaline potassium ferricyanide. Reaction: 2K3Fe(CN)6 + 2KOH + H2O2 → 2K4Fe(CN)6 + 2H2O + O2
Why Is Hydrogen Peroxide Stored in Plastic Containers?
Hydrogen peroxide decomposes when exposed to sunlight, this process is catalysed by traces of alkali metals. Therefore, H2O2 is stored in wax-lined glass or plastic containers and kept in the dark.
It should also be kept away from dust particles because dust can induce explosive decomposition of this compound.
How to Determine the Concentration of Hydrogen Peroxide?
Volume Strength of H2O2
10 Vol H2O2 means 1 mL of H2O2 gives 10 mL of O2 at STP.
Weight Percentage [w/w%]
Let us consider 10 Vol H2O2
2H2O2 → 2H2O + O2
→ 68g of H2O2 gives 22.4 L of O2 at STP.
→ 10 L of O2 is given by
Amount of hydrogen peroxide = 10 × 68/22.4 = 30.36 g
1000 mL of H2O2 → 30.36 g
100 mL → ?
= 30.36%
100 Vol H2O2 or 3.036%(w/w) H2O2 is known as Perhydrol.
Molarity of H2O2
To calculate the molarity, let us consider 10 Vol H2O2
Molarity of hydrogen peroxide = 30.36/34 = 0.89 M.
Normality of H2O2
Let us consider 10 Vol H2O2
Also Read: What Is Hydrogen Peroxide Used for?
Hydrogen Peroxide – Video Lesson

Frequently Asked Questions (FAQs)
What is hydrogen peroxide used for?
State three physical properties of hydrogen peroxide.
It is a pale blue colour in its pure form.
The viscosity of H2O2 is slightly more than water.
The boiling point of hydrogen peroxide is 150.2°C.
What is the oxidation of oxygen in hydrogen peroxide?
Give an example of a reaction in which hydrogen peroxide is an oxidising agent.
NaNO2 + H2O2 → NaNO3 + H2O
What is the product of the reaction between ozone and hydrogen peroxide?
O3 + H2O2 → H2O + 2O2

nice article.