
Sodium Thiosulphate, also called thiosulfuric acid or disodium salt, is an inorganic salt that is also available in pentahydrates. This chemical substance has a chemical formula of Na2S2O3. It appears as a bright white colourless crystal or even in powder form. The substance is known to possess alkaline nature when decomposed to sulphide and sulfate in the air.
Sodium thiosulfate readily dissolves in water giving thiosulfate ions, which is one of the useful reducing agents. The Copper (II) sulfate dissolves to give the cupric ion; in regard to a redox reaction with the thiosulfate, the cupric particles act like oxidising agents.
Properties of Sodium Thiosulphate
Sodium Thiosulphate possesses various chemical and physical properties as given below:
- Sodium thiosulphate appears as a white translucent, colourless crystal and is an inorganic compound.
- It is a water-soluble substance and is also soluble in the oil of turpentine but not in alcohol.
- The substance has a melting point of about 48 to 52 C.
- This chemical substance is highly stable in nature and is said to be incompatible with some strong oxidising agents and strong acids.
- Thiosulfate anion readily reacts with the dilute acids producing sulphur, sulphur dioxide and also water.
- The chemical has a density measuring about 1.667 g/mL.
Structure of Sodium Thiosulphate
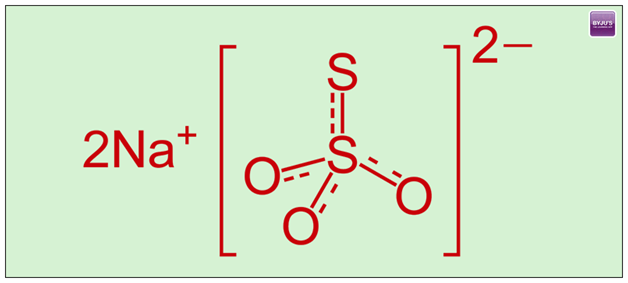
Sodium thiosulphate has a chemical formula of Na2S2O3 and a molar mass of about 158.11 g/mol. The substance well exists in the form of pentahydrate salts (Na2S2O3.5H2O), having a molar mass measuring about 248.18 g/mol. Sodium thiosulphate is an ionic compound which consists of two cations of sodium atom (Na+) and a negatively charged anion of thiosulfate (S2O3-). Here, the central atom consisting of sulphur bonds to the three oxygen atoms and also another atom of sulphur, all these through a single and also double bonds possessing resonance character. The solid also exists in a monoclinic crystalline structure.
The thiosulfate anion is usually a tetrahedral structure and is obtained by the replacement of one of the atoms of oxygen by the use of a sulfur atom in the sulfate anion.
Uses and Applications of Sodium Thiosulphate
We find various applications of sodium thiosulphate in different fields such as medicine, photography, gold extraction and many other areas. Its other uses are as given below:
- Sodium thiosulfate is used in the manufacture of patinas
- In industries, the chemical is used for the dechlorination of small water bodies like ponds, aquariums, etc.
- In photography, the chemical is used as a fixing agent to dissolve the silver salts from the negatives
- The chemical can be used as a cleansing agent when dissolved in a vast quantity of warm water
- It is well used as an antidote agent concerning cyanide poisoning
- In the medical field, it is employed in pharmaceutical preparations, such as anionic surfactant aiding in dispersion
Apart from the above uses, the chemical substance also finds its applications in water treatment, leather tanning, neutralising bleach, gold extraction, photographic film processing, and also in chemical heating pads.

Comments