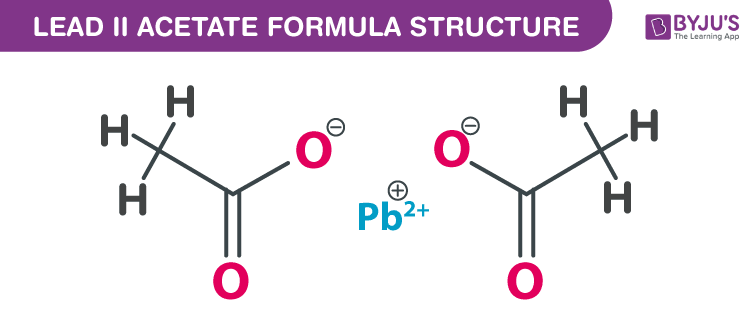
Lead (II) acetate formula, also named as Salt of Saturn formula or Plumbous acetate formula is discussed in this article. It is toxic and denser than water. The molecular or chemical formula of Lead (II) acetate is Pb(C2H3O2)2.
Salt of Saturn is a white to grey coloured crystalline solid. It has a slight acetic smell and is sweet to taste. It can be synthesized by boiling elemental lead in acetic acid (CH3COOH) and hydrogen peroxide (H2O2). It can also be prepared through a single displacement reaction. This reaction is made to occur between the lead metal and copper acetate.
Lead (II) acetate Formula Structure
Properties Of Lead (II) acetate Formula
| Chemical formula | Pb(C2H3O2)2 |
| Molecular weight | 325.29 g/mol (anhydrous) |
| Density | 3.25 g/cm3 (anhydrous) |
| Boiling point | Decomposes |
| Melting point | 280°C |
safety measures and applications
- When this chemical compound comes in contact it irritates eyes, mucous membranes, and skin.
- It can be toxic when inhaled, ingested, or through skin absorption.
- It is widely used in waterproofing, hair dyes, insecticides, dyes, antifouling paints, etc.
To learn more about Lead (II) acetate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Salt of Saturn for free.
Comments