Lead IV Oxide is a hexagonal dark brown crystalline powder that is insoluble in water. This chemical is widely used in the manufacturing of matches, dyes, electrodes, pyrotechnics, positive plates of lead-acid batteries and explosives. It is an extremely strong oxidant and is harmful and poisonous in nature.
Properties Of Lead IV Oxide
| Chemical formula | PbO2 |
| Chemical names | Plumbic oxide
Plattnerite |
| Molecular weight | 239.198 g/mol |
| Density | 9.38 g/cm3 |
| Melting point | 290 °C |
Lead IV Oxide Structural Formula
The structural formula for Lead IV Oxide is as shown in the figure below.
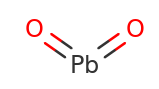
Lead IV Oxide features two compounds Pb4+ and O2− in the ratio 1:2 and hence the formula is PbO2. To form each Pb4+ it requires two negative oxide ions O2− to balance out total positive and total negative charge on it.
Stay tuned with BYJU’S to know more scientific information!!
Comments